BGT226 free base
Modify Date: 2024-01-05 09:11:49
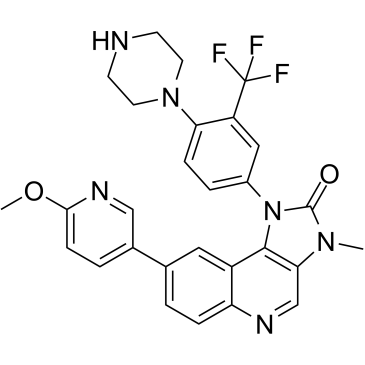
BGT226 free base structure
|
Common Name | BGT226 free base | ||
|---|---|---|---|---|
| CAS Number | 915020-55-2 | Molecular Weight | 534.53 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 713.3±70.0 °C at 760 mmHg | |
| Molecular Formula | C28H25F3N6O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 385.2±35.7 °C | |
Use of BGT226 free baseBGT226 (NVP-BGT226) is a PI3K (with IC50s of 4 nM, 63 nM and 38 nM for PI3Kα, PI3Kβ and PI3Kγ)/mTOR dual inhibitor which displays potent growth-inhibitory activity against human head and neck cancer cells[1][2]. |
| Name | 8-(6-methoxypyridin-3-yl)-3-methyl-1-[4-piperazin-1-yl-3-(trifluoromethyl)phenyl]imidazo[4,5-c]quinolin-2-one |
|---|---|
| Synonym | More Synonyms |
| Description | BGT226 (NVP-BGT226) is a PI3K (with IC50s of 4 nM, 63 nM and 38 nM for PI3Kα, PI3Kβ and PI3Kγ)/mTOR dual inhibitor which displays potent growth-inhibitory activity against human head and neck cancer cells[1][2]. |
|---|---|
| Related Catalog | |
| Target |
PI3Kα:4 nM (IC50) PI3Kβ:63 nM (IC50) PI3Kγ:38 nM (IC50) mTOR Autophagy |
| In Vitro | BGT226 shows significant growth inhibition or signal blockage profiles compared with LY294002 and Rapamycin. BGT226 (10-10000 nM) inhibits FaDu and OECM1 cells growth with IC50s of 23.1±7.4 and 12.5±5.1 nM, respectively[2]. The expression levels of p-mTOR Ser2481 are decreased in BGT226-treated cell lines (200 nM; 24 hours) and both p-AKT Ser473 and p-mTOR Ser2448 are also decreased in BGT226-treated cell lines[2]. Cell Viability Assay[2] Cell Line: FaDu cells; OECM1 cells Concentration: 10, 100, 1000, 10000 nM Incubation Time: Result: Inhibited FaDu and OECM1 cells growth with IC50s of 23.1±7.4 and 12.5±5.1 nM, respectively. Western Blot Analysis[2] Cell Line: FaDu cells; OECM1 cells Concentration: 200 nM Incubation Time: 24 hours Result: p-mTOR Ser2481 expression levels decreased, and both p-AKT Ser473 and p-mTOR Ser2448 expression levels also decreased. |
| In Vivo | BGT226 (2.5 and 5 mg/kg; oral administration for 21 days in male athymic mice) causes 34.7% and 76.1% reduction of the tumor growth on day 21 compared with control[2]. Animal Model: Male athymic mice (strain BALB/cAnN.Cg-Foxn1nu/CrlNarl) with FaDu cell xenografted mouse model[2] Dosage: 2.5 and 5 mg/kg Administration: Oral administration; 21 days Result: Caused 34.7% and 76.1% reduction of the tumor growth. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 713.3±70.0 °C at 760 mmHg |
| Molecular Formula | C28H25F3N6O2 |
| Molecular Weight | 534.53 |
| Flash Point | 385.2±35.7 °C |
| Exact Mass | 534.199097 |
| PSA | 77.21000 |
| LogP | 3.41 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.628 |
| 8-(6-Methoxy-3-pyridinyl)-3-methyl-1-[4-(1-piperazinyl)-3-(trifluoromethyl)phenyl]-1,3-dihydro-2H-imidazo[4,5-c]quinolin-2-one |
| 2H-Imidazo[4,5-c]quinolin-2-one, 1,3-dihydro-8-(6-methoxy-3-pyridinyl)-3-methyl-1-[4-(1-piperazinyl)-3-(trifluoromethyl)phenyl]- |
| BGT226 free base |
| BGT-226 free base |
| 8-(6-methoxypyridin-3-yl)-3-methyl-1-[4-(piperazin-1-yl)-3-(trifluoromethyl)phenyl]-1,3-dihydro-2H-imidazo[4,5-c]quinolin-2-one |
| 8-(6-Methoxy-pyridin-3-yl)-3-methyl-1-(4-piperazin-1-yl-3-trifluoromethyl-phenyl)-1,3-dihydro-imidazo[4,5-c]quinolin-2-one |
| UNII-ZXE7F2GMJJ |