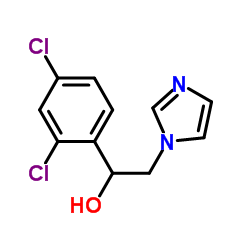
99592-32-2
| 中文名 | 舍他康唑 |
|---|---|
| 英文名 | 1-[2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole |
| 中文别名 | 1-[2-(7-氯苯并[b]噻吩-3-基)甲氧基-2-(2,4-二氯苯)乙基]-1H-咪唑硝酸盐 |
| 英文别名 |
Sertaconazolum
Sertaconazol 1H-Imidazole, 1-[2-[(7-chlorobenzo[b]thien-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]- 1-{2-[(7-Chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole T56 BSJ IG D1OYR BG DG&1- AT5N CNJ Sertaconazole (INN) MFCD00868881 Sertaconazolum [Latin] sertaconazole Ertaczo Sertaconazol [Spanish] 1-{2-[(7-chloro-3-benzo[b]thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole 1-[2-(7-chlorobenzo[b]thiophene-3-yl-methoxy)-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole 7-Chloro-3-[1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethoxy-methyl]benzo[b]thiophene 1-[2-[(7-chlorobenzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)-ethyl]imidazole (±)-1-[2,4-Dichloro-β-[(7-chlorobenzo[b]thien-3-yl)methoxy]phenethyl]imidazole FI-7045 7-chloro-3-[1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethoxymethyl]benzo[b]thiophene 1-{2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl}imidazole |
| 描述 | 舍他康唑(FI7056游离碱)是一种广谱局部抗真菌剂,通过激活p38-COX-2-PGE2途径发挥抗炎活性。舍他康唑也是一种微管抑制剂,具有抗增殖作用,诱导细胞凋亡和自噬,还可以抑制细胞迁移[1][2][3][4]。 |
|---|---|
| 相关类别 | |
| 体外研究 | Sertaconazole(0.03-40µg/mL;24小时)抑制150株酵母菌,其中包括六种念珠菌,算术平均MIC为0.77µg/mL[1]。舍他康唑(1µg/mL;5、10、30、60分钟)以时间依赖性方式激活p38 MAP激酶[2]。Sertaconazole(1,2µg/mL;6,8或24小时)通过COX-2在角质形成细胞中增加PGE2的两倍释放,这依赖于p38激活[2]。西康唑(10、20、30、40µM;24小时)通过解聚间期微管和纺锤体微管,诱导强烈的有丝分裂阻滞,从而诱导染色体聚集缺陷并产生抗增殖作用[3]。Sertaconazole(20,40µM;24小时)通过p53途径诱导HeLa细胞凋亡[3]。Sertaconazole(20、30µM;24、48和72小时)以浓度依赖性方式抑制HeLa细胞的迁移[3]。舍他康唑(15,30µM;24小时)诱导A549、H460细胞自噬[4]。细胞存活率测定[1]细胞系:白念珠菌、吉列蒙念珠菌、克鲁西念珠菌、副硅氧烷、热带念珠菌、无毛念珠菌浓度:0.03-40µg/m孵育时间:24小时结果:对150株酵母菌(六种念珠菌),其中包括白念珠菌,吉列蒙弧菌、克鲁斯弧菌、副西罗弧菌、热带念珠杆菌、无毛弧菌,其算术平均MIC值分别为1.02、0.51、0.38,分别为0.31、1.67和0.78µg/mL。Western印迹分析[2]细胞系:HaCaT细胞浓度:1µg/mL孵育时间:5、10、30、60分钟结果:显示p38 MAP激酶和Hsp27的激活活性呈时间依赖性。Western印迹分析[2]细胞系:HaCaT细胞浓度:1,2µg/mL孵育时间:6或8小时结果:诱导COX-2表达50%,导致PGE2释放增加两倍。Western印迹分析[2]细胞系:siRNA转染的HaCaT细胞(无p38 MAP激酶表达)浓度:1µg/mL孵育时间:24小时结果:介导的PGE2诱导依赖于p38激活。细胞增殖试验[3]细胞系:HeLa、HEK-293、MCF-7、A549细胞浓度:0-100µM孵育时间:24小时结果:显示抗增殖活性,对HeLa,HEK-29 3、A549和MCF-9细胞的IC50分别为38、45.1、41.5和40.8µM。在浓度超过30μM时表现出有丝分裂阻滞活性和诱导细胞死亡,但有丝分裂细胞数量没有显著增加。解聚的间期微管和纺锤体微管导致染色体一致性缺陷。凋亡分析[3]细胞系:HeLa细胞浓度:10、20、40µM孵育时间:24小时结果:在浓度为10、20和40μM时,分别诱导约5%、10%和21%的细胞凋亡。Western Blot分析[3]细胞系:A549细胞浓度:20,40µM孵育时间:24小时结果:通过p53途径诱导凋亡,p53表达分别从30%到50%和95%,p21表达分别从11%到39%和40%。导致p53的两个直接转录靶标Noxa和Puma过度表达。细胞迁移试验[3]细胞系:HeLa细胞浓度:20、30µM孵育时间:24、48和72 h结果:在低于其IC50的浓度下抑制HeLa的迁移,其浓度依赖性。细胞自噬测定[4]细胞系:A549、H460细胞浓度:15、30µM孵育时间:24小时结果:内源性LC3点状细胞和LC3强度增加,这表明A549和H460中诱导了自噬。 |
| 体内研究 | 舍他康唑(1%(w/v);应用于左耳一次)抑制TPA诱导的CD-1小鼠耳水肿[2]。动物模型:CD-1小鼠(TPA诱导的耳水肿模型)[2]。剂量:1%(w/v)给药:应用于左耳,一次。结果:通过介导PGE2的释放,小鼠的炎症反应明显减轻。 |
| 参考文献 |
| 密度 | 1.4±0.1 g/cm3 |
|---|---|
| 沸点 | 614.1±55.0 °C at 760 mmHg |
| 分子式 | C20H15Cl3N2OS |
| 分子量 | 437.770 |
| 闪点 | 325.2±31.5 °C |
| 精确质量 | 435.997070 |
| PSA | 55.29000 |
| LogP | 7.49 |
| 蒸汽压 | 0.0±1.7 mmHg at 25°C |
| 折射率 | 1.675 |
| 储存条件 | -20°C |
| 稳定性 | 硝酸舍他康唑(Sertaconazole Nitrate):C20 H15C13N2OS?HNO3。[99592-39-9]。白色结晶性粉末,无臭。易溶于乙醇(1.7%)或氯仿(1.5%),微溶于丙酮(0.95%),极微溶于正辛醇(0.069%),几不溶于水(<0.01%)。pKb7.26。熔点158~160℃。UV最大吸收(甲醇):302.3(A1cm1%79.8),292.9,260.3nm。 |
| 分子结构 | 1、 摩尔折射率:114.53 2、 摩尔体积(cm3/mol):304.8 3、 等张比容(90.2K):304.8 4、 表面张力(dyne/cm):51.4 5、 极化率(10-24cm3):45.40 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:0 3.氢键受体数量:3 4.可旋转化学键数量:6 5.互变异构体数量:无 6.拓扑分子极性表面积55.3 7.重原子数量:27 8.表面电荷:0 9.复杂度:488 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:1 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1.熔点:146-147℃。 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 危害码 (欧洲) | Xi |
|---|---|
| 安全声明 (欧洲) | S22-S24/25 |
| WGK德国 | 2 |
| RTECS号 | KM6557000 |
|
~% 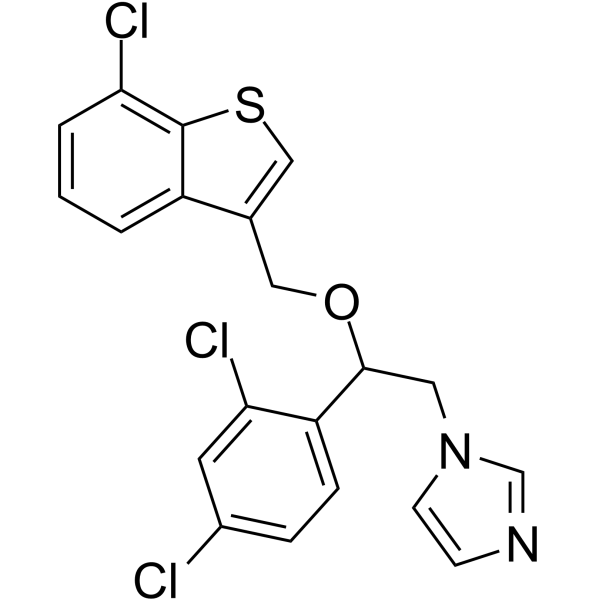
99592-32-2 |
| 文献:European Journal of Medicinal Chemistry, , vol. 21, # 4 p. 329 - 332 |
| 上游产品 2 | |
|---|---|
| 下游产品 0 | |