CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
DO1800000
-
CHEMICAL NAME :
-
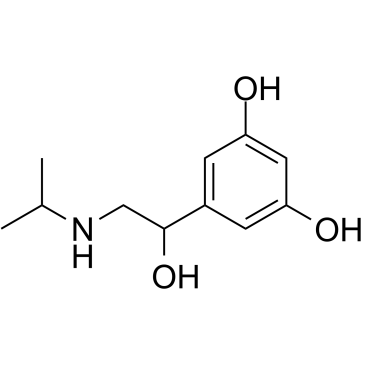
Benzyl alcohol, 3,5-dihydroxy-alpha-((isopropylamino)methyl)-
-
CAS REGISTRY NUMBER :
-
586-06-1
-
LAST UPDATED :
-
199709
-
DATA ITEMS CITED :
-
13
-
MOLECULAR FORMULA :
-
C11-H17-N-O3
-
MOLECULAR WEIGHT :
-
211.29
-
WISWESSER LINE NOTATION :
-
QR CQ EYQ1MY
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
286 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - tremor Cardiac - arrhythmias (including changes in conduction) Vascular - BP elevation not characterized in autonomic section
-
REFERENCE :
-
AJMSA9 American Journal of the Medical Sciences. (Slack Inc., 6900 Grove Rd., Thorofare, NJ 08086) New series: V.1- 1841- Volume(issue)/page/year: 291,168,1986
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3370 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 8,353,1966
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
67200 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 8,353,1966
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>8130 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 8,353,1966
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
240 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - sympathomimetic
-
REFERENCE :
-
APTOA6 Acta Pharmacologica et Toxicologica. (Copenhagen, Denmark) V.1-59, 1945-86. For publisher information, see PHTOEH Volume(issue)/page/year: 31,33,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
295 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
USXXAM United States Patent Document. (U.S. Patent Office, Box 9, Washington, DC 20231) Volume(issue)/page/year: #4011258
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
86 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Cardiac - pulse rate increase, without fall in BP Skin and Appendages - hair
-
REFERENCE :
-
APTOA6 Acta Pharmacologica et Toxicologica. (Copenhagen, Denmark) V.1-59, 1945-86. For publisher information, see PHTOEH Volume(issue)/page/year: 38,474,1976
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
125 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 8,353,1966
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
30 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 8,353,1966
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
3110 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 8,353,1966
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
81300 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 8,353,1966 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
800 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
REFERENCE :
-
RDCNBM Reproduccion. (Madrid, Spain) V.1-7, 1974-83. Discontinued. Volume(issue)/page/year: 5,31,1981 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X5007 No. of Facilities: 35 (estimated) No. of Industries: 1 No. of Occupations: 1 No. of Employees: 476 (estimated) No. of Female Employees: 255 (estimated)
|