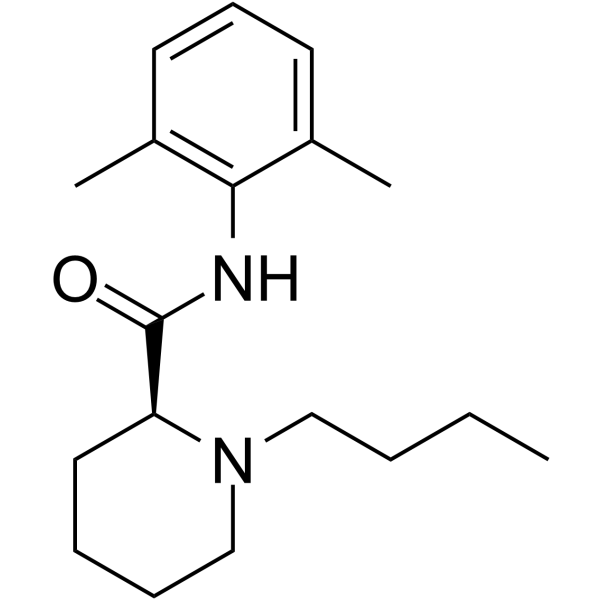
27262-47-1
| 中文名 | 左布比卡因 |
|---|---|
| 英文名 | levobupivacaine |
| 中文别名 |
1-丁基-N-(2,6-二甲基苯基)-哌啶-2-甲酰胺
LEVOBUPIVACAINE 左布比卡因 左旋布比卡因 左布比咔因 |
| 英文别名 |
2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)-, (2S)-
(2S)-1-Butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide L-(-)-Bupivacaine 2',6'-Pipecoloxylidide,1-butyl-,L-(-) 1-Butyl-N-(2,6-dimethylphenyl)-piperidine-2-carboxamide (-)-Bupivacaine Levobupivacaine 1-butyl-piperidine-2-carboxylic acid-(2,6-dimethyl-anilide) (S)-(-)-Bupivacaine (S)-Bupivacaine (2S)-1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide |
| 描述 | 左旋布比卡因((S)-(-)-布比卡因)是一种长效酰胺类局部麻醉剂。左旋布比卡因通过可逆阻断神经元钠通道发挥麻醉和镇痛作用。左布比卡因可抑制心血管和其他组织中的脉冲传递和传导,具有一定的心脏和中枢神经系统毒性。左布比卡因在体内由肝细胞色素P450(CYP450)酶代谢。左旋布比卡因还可通过miR-489-3p/SLC7A11信号在胃癌中诱导铁下垂[1][2][3]。 |
|---|---|
| 相关类别 | |
| 靶点 |
Sodium channels, Ferroptosis[1][2] |
| 体外研究 | 左旋布比卡因(0-4 mM;24小时)不影响GES-1细胞的存活率,但抑制HGC27和SGC7901细胞的存活[2]。左旋布比卡因(2mM;24、48或72小时)增强Erastin诱导的对HGC27和SGC7901细胞活性的抑制作用;诱导Fe2+、铁和脂质ROS的水平[2]。左旋布比卡因(2mM;24h)增强HGC27和SGC7901细胞中miR-489-3p的表达,增加Fe2+和铁的水平[2]。细胞活力测定[2]细胞系:GES-1、HGC27和SGC790浓度:0、0.5、1、2和4 mM孵育时间:24 h结果:不影响正常胃上皮GES-2细胞系的活力,但以剂量依赖性方式抑制HGC27与SGC7901细胞的活力。细胞存活率测定[2]细胞系:HGC27和SGC7901(与5μM erastin孵育)浓度:2mM孵育时间:24、48或72小时结果:erastin对HGC27及SGC791细胞存活率的抑制作用增强;诱导Fe2+、铁和脂质ROS的水平。RT-PCR[2]细胞系:HGC27和SGC7901(与5μM erastin孵育)浓度:2mM孵育时间:24h结果:增强了HGC27细胞和SGC79 01细胞中miR-489-3p的表达,增加了Fe2+和铁的水平。 |
| 体内研究 | 左旋布比卡因(40μmol/kg;IP;每天一次,持续25天)显著抑制SGC7901细胞生长,并增强脂质ROS的积累[2]。左旋布比卡因(5或36 mg/kg;IP;单剂量)增加部分癫痫发作的潜伏期,并在低剂量下防止全身性癫痫发作的发生;降低N-甲基-d-天冬氨酸(NMDA)诱导癫痫发作的潜伏期,并在高剂量下增加癫痫发作的严重程度[3]。动物模型:CD1小鼠(30-35g;通过注射NMDA诱导癫痫发作)[3]剂量:5或36mg/kg给药:IP;单剂量结果:5mg/kg增加了部分癫痫发作的潜伏期,并防止了全身性癫痫发作的发生;降低NMDA诱发癫痫的潜伏期,并增加36mg/kg时的癫痫严重程度。动物模型:SCID裸鼠(6-8周;皮下注射5×106 SGC7901细胞)[2]剂量:40μmol/kg给药:IP;结果:显著抑制SGC7901细胞生长,增强脂质ROS积累。 |
| 参考文献 |
| 密度 | 1.0±0.1 g/cm3 |
|---|---|
| 沸点 | 423.4±45.0 °C at 760 mmHg |
| 熔点 | 254ºC (dec.)(lit.) |
| 分子式 | C18H28N2O |
| 分子量 | 288.428 |
| 闪点 | 209.9±28.7 °C |
| 精确质量 | 288.220154 |
| PSA | 32.34000 |
| LogP | 3.64 |
| 外观性状 | 白色固体 |
| 蒸汽压 | 0.0±1.0 mmHg at 25°C |
| 折射率 | 1.547 |
| 储存条件 | 库房通风低温干燥,与食品原料类分开存放 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 危害码 (欧洲) | Xn: Harmful;T+: Very toxic; |
|---|---|
| 危险品运输编码 | UN 2811 6 |
| 海关编码 | 2933399090 |
| 海关编码 | 2933399090 |
|---|---|
| 中文概述 | 2933399090. 其他结构含非稠合吡啶环的化合物. 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途, 乌洛托品请注明外观, 6-己内酰胺请注明外观, 签约日期 |
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |