10118-90-8
| 中文名 | 米诺环素 |
|---|---|
| 英文名 | minocycline |
| 中文别名 |
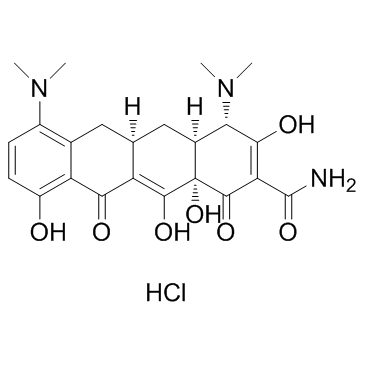
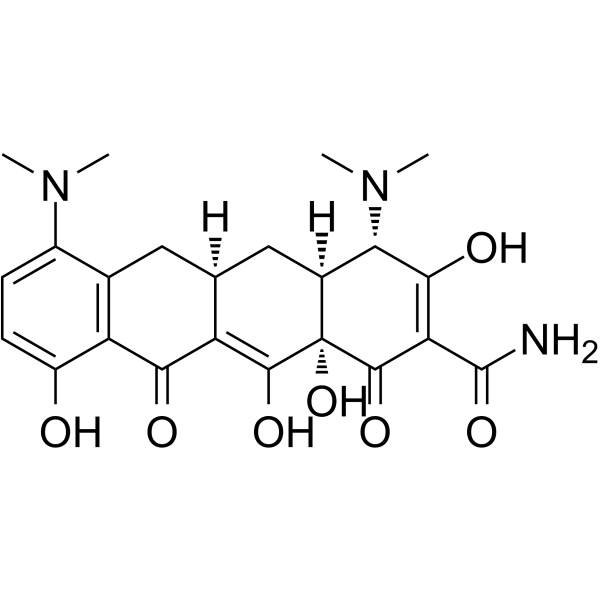
4,7-双(二甲氨基)-1,4,4a,5,5a,6,11,12a-八氢-3,10,12,12a-四羟基-1,11-二氧-2-并四苯甲酰胺
二甲胺四环素 7-二甲胺基-6-去甲基-6-去氧四环素 |
| 英文别名 |
(4S,4aS,5aR,12aS)-4,7-Bis(dimethylamino)-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-2-tetracenecarboximidic acid
Minocyclin Minocin 7-dimethylamino-6-demethyl-6-deoxytetracycline (4S,4aS,5aR,12aS)-4,7-Bis(dimethylamino)-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-2-tetracenecarboxamide 4S-(4a,4aa,5aa,12aa)-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacene carboxamide Minocyclinum [4S-(4a,4aa,5aa,12aa)]-4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide 2-Naphthacenecarboximidic acid, 4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-, (4S,4aS,5aR,12aS)- Apo-Minocycline (4S,4aS,5aR,12aR)-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide (4S,4aS,5aR,12aS)-4,7-bis(dimethylamino)-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide 2-Naphthacenecarboxamide, 4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-, (4S,4aS,5aR,12aS)- Minocycline Arestin 7-(N,N-dimethylamino)sancycline Aknemin Minocyclinum [INN-Latin] EINECS 237-099-7 Dynacin Minociclina MFCD00083669 |
| 描述 | 米诺环素是一种口服活性、强效和BBB渗透的半合成四环素抗生素。米诺环素是一种缺氧诱导因子(HIF)-1α抑制剂。米诺环素具有抗癌、抗炎和谷氨酸拮抗作用。米诺环素可减少谷氨酸神经传递,并具有神经保护和抗抑郁作用。米诺环素通过与细菌核糖体的30S亚单位结合抑制细菌蛋白质合成,从而产生抑菌作用[1][2][3][4][5][6][7]。 |
|---|---|
| 相关类别 | |
| 靶点 |
L-type calcium channel |
| 体外研究 | 米诺环素(0-100μM,24-72小时)抑制卵巢癌细胞系(OVCAR-3、SKOV-3和A2780)的增殖和克隆形成活性[3]。米诺环素(0-100μM,24-48小时)通过抑制细胞周期蛋白和抑制DNA掺入来阻止细胞周期[3]。米诺环素(0-100μM,72小时)诱导卵巢癌细胞系中的细胞凋亡[3]。米诺环素显示出直接的神经元保护,这种保护模式可能与线粒体完整性和细胞色素c的保存有关,随后抑制caspase依赖性和caspase非依赖性细胞死亡[2]。米诺环素导致缺氧诱导因子(HIF)-1α的抑制,伴随着p53蛋白水平的上调和AKT/mTOR/p70S6K/4E-BP1途径的失活[6]。细胞增殖试验[3]细胞系:人卵巢癌细胞系(OVCAR-3、SKOV-3和A2780)和原代细胞(HEK-293、HMEC、HUVEC、ATCC)浓度:0、1、10、50和100μM孵育时间:24、48或 |
| 体内研究 | 米诺环素(0-30mg/kg,口服,每日4周)抑制雌性裸鼠OVCAR-3肿瘤生长[3]。在大剂量腹腔注射时,米诺环素(IP)是脑缺血动物模型中的有效神经保护剂[1]。米诺环素(0-40mg/kg,IP,一次)可显著减轻甲基苯丙胺诱导的小鼠过度运动和行为敏化[2]。米诺环素(3和10 mg/kg,静脉注射一次)可有效减少暂时性大脑中动脉闭塞模型(TMCAO)中的梗死面积[1]。二甲胺四环素(3-10 mg/kg,静脉注射一次)的血清水平(3 mg/kg)与标准200 mg剂量后的人体水平相似[1]。米诺环素减轻大鼠缺血诱发的室性心律失常。这种效应可能与PI3K/Akt信号通路、线粒体KATP通道和L型Ca2+通道的激活有关[7]。动物模型:雌性裸鼠(6周龄,每组9只,将OVCAR-3细胞经皮下注射至左侧腹壁 |
| 参考文献 |
| 密度 | 1.6±0.1 g/cm3 |
|---|---|
| 沸点 | 803.3±65.0 °C at 760 mmHg |
| 分子式 | C23H27N3O7 |
| 分子量 | 457.476 |
| 闪点 | 439.6±34.3 °C |
| 精确质量 | 457.184906 |
| PSA | 164.63000 |
| LogP | -0.65 |
| 外观性状 | 亮黄色-橙色非晶形的固体 |
| 蒸汽压 | 0.0±3.0 mmHg at 25°C |
| 折射率 | 1.718 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
~% 
10118-90-8 |
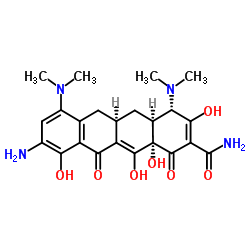
| 文献:WO2010/33939 A1, ; Page/Page column 36 ; |
|
~66% 
10118-90-8 |
| 文献:The Journal of organic chemistry, , vol. 67, # 14 p. 5025 - 5027 |
| 上游产品 3 | |
|---|---|
| 下游产品 1 | |