33564-31-7
| 中文名 | 醋酸双氟拉松 |
|---|---|
| 英文名 | diflorasone diacetate |
| 中文别名 |
二乙酸二氟拉松酯
双氟拉松乙酸酯 |
| 英文别名 |
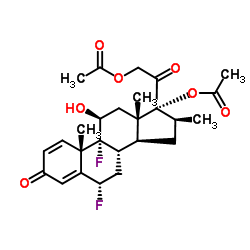
[2-[(6S,8S,9R,10S,11S,13S,14S,16S,17R)-17-acetyloxy-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] acetate
(6S,8S,9R,10S,11S,13S,14S,16S,17R)-17-[(acetyloxy)acetyl]-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl acetate Diflorasone diacetate (6α,11β,16β)-6,9-Difluoro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl diacetate acétate de (6S,8S,9R,10S,11S,13S,14S,16S,17R)-17-[(acétyloxy)acétyl]-6,9-difluoro-11-hydroxy-10,13,16-triméthyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodécahydro-3H-cyclopenta[a]phénanthrén-17-yle PSORCON 6a,9-Difluoro-11b,17,21-trihydroxy-16b-methylpregna-1,4-diene-3,20-dione 17,21-Diacetate 6a-Fluorobetamethasone-17,21 Diacetate Pregna-1,4-diene-3,20-dione, 17,21-bis(acetyloxy)-6,9-difluoro-11-hydroxy-16-methyl-, (6α,11β,16β)- Diflorasone diacetate [USAN:JAN] MFCD00079159 EINECS 251-575-1 (6S,8S,9R,10S,11S,13S,14S,16S,17R)-17-[(Acetyloxy)acetyl]-6,9-difluor-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-ylacetat Diflorasone diacetate (JAN/USP) |
| 描述 | 二醋酸地氟松是一种抗炎类固醇化合物,用作局部或局部药物。二醋酸地氟松用于皮肤疾病,以控制皮质类固醇反应性皮肤病[1]。 |
|---|---|
| 相关类别 | |
| 参考文献 |
| 密度 | 1.3±0.1 g/cm3 |
|---|---|
| 沸点 | 585.1±50.0 °C at 760 mmHg |
| 熔点 | 47-49 °C(lit.) |
| 分子式 | C26H32F2O7 |
| 分子量 | 494.525 |
| 闪点 | 307.6±30.1 °C |
| 精确质量 | 494.211609 |
| PSA | 106.97000 |
| LogP | 3.10 |
| 外观性状 | 晶体 |
| 蒸汽压 | 0.0±3.7 mmHg at 25°C |
| 折射率 | 1.546 |
| 储存条件 | Refrigerator |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:1 3.氢键受体数量:9 4.可旋转化学键数量:6 5.互变异构体数量:2 6.拓扑分子极性表面积107 7.重原子数量:35 8.表面电荷:0 9.复杂度:1050 10.同位素原子数量:0 11.确定原子立构中心数量:9 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:未确定 2. 密度(g/mL,20℃):未确定 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):未确定 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,KPa):未确定 7. 折射率:未确定 8. 闪点(ºC):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(Pa,20ºC):未确定 12. 饱和蒸气压(KPa,20ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:未确定 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 符号 |


GHS07, GHS08 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H312 + H332-H351 |
| 警示性声明 | P261-P280 |
| 危害码 (欧洲) | Xn:Harmful; |
| 风险声明 (欧洲) | R20/21/22;R40 |
| 安全声明 (欧洲) | S22-S36 |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 3 |
| RTECS号 | TU3722000 |
| 海关编码 | 2942000000 |
| 海关编码 | 2942000000 |
|---|