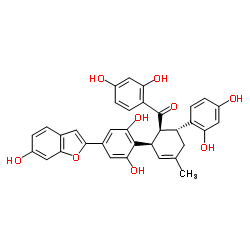
87085-00-5
| Name | Mulberrofuran G |
|---|---|
| Synonyms |
(3aR,8aS,13bR,13cS)-8a-(2,4-Dihydroxyphenyl)-6-(6-hydroxy-1-benzofuran-2-yl)-2-methyl-1,8a,13b,13c-tetrahydro-3aH-benzo[3,4]isochromeno[1,8-bc]chromene-4,11-diol
albanol A Mulberrofuran G 3aH-Benzo[c][1]benzopyrano[4,3,2-ij][2]benzopyran-4,11-diol, 8a-(2,4-dihydroxyphenyl)-1,8a,13b,13c-tetrahydro-6-(6-hydroxy-2-benzofuranyl)-2-methyl-, (3aR,8aS,13bR,13cS)- |
| Description | Mulberrofuran G protects ischemic injury-induced cell death via inhibition of NOX4-mediated ROS generation and ER stress[1]. Mulberrofuran G shows moderate inhibiting activity of hepatitis B virus (HBV) DNA replication with the |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 695.1±55.0 °C at 760 mmHg |
| Molecular Formula | C34H26O8 |
| Molecular Weight | 562.57 |
| Flash Point | 374.2±31.5 °C |
| Exact Mass | 562.162781 |
| PSA | 132.75000 |
| LogP | 6.75 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.745 |
| Storage condition | 2-8℃ |
| Hazard Codes | Xi |
|---|
|
~% 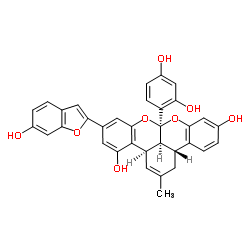
87085-00-5 |
| Literature: Fukai, Toshio; Hano, Yoshio; Hirakura, Kazuhiro; Nomura, Taro; Uzawa, Jun; Fukushima, Kazutaka Heterocycles, 1984 , vol. 22, # 3 p. 473 - 477 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |