1092939-17-7
| Name | ruxolitinib phosphate |
|---|---|
| Synonyms |
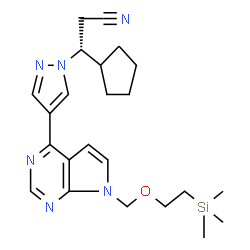
(3R)-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propionitrile phosphate
(R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile phosphate Ruxolitinib phosphate (3R)-3-Cyclopentyl-3-[4-(1H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile phosphate (1:1) 1H-Pyrazole-1-propanenitrile, β-cyclopentyl-4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-, (βR)-, phosphate (1:1) Jakafi Ruxolitinib (phosphate) |
| Description | Ruxolitinib phosphate is a potent JAK1/2 inhibitor with IC50s of 3.3 nM/2.8 nM, respectively, showing more than 130-fold selectivity over JAK3. |
|---|---|
| Related Catalog | |
| Target |
JAK2:2.8 nM (IC50) JAK1:3.3 nM (IC50) Tyk2:19 nM (IC50) JAK3:428 nM (IC50) |
| In Vitro | Ruxolitinib (INCB018424) potently and selectively inhibits JAK2V617F-mediated signaling and proliferation. Ruxolitinib inhibits the growth of HEL cells with EC50 of 186 nM. Ruxolitinib markedly increases apoptosis in Ba/F3-EpoR-JAK2V617F cell system, and inhibits hematopoietic progenitor cell proliferation in primary MPN patient samples[1]. |
| In Vivo | Ruxolitinib (180 mg/kg, p.o.) reduces the tumor burden of mice inoculated with JAK2V617F-expressing cells without causing anemia or lymphopenia[1]. |
| Cell Assay | Cells are seeded at 2000/well of white bottom 96-well plates, treated with compounds from DMSO stocks (0.2% final DMSO concentration), and incubated for 48 hours at 37°C with 5% CO2. Viability is measured by cellular ATP determination using the Cell-Titer Glo luciferase reagent or viable cell counting. Values are transformed to percent inhibition relative to vehicle control, and IC50 curves are fitted according to nonlinear regression analysis of the data using PRISM GraphPad. |
| Animal Admin | Mice are fed standard rodent chow and provided with water ad libitum. Ba/F3-JAK2V617F cells (105 per mouse) are inoculated intravenously into 6- to 8-week-old female BALB/c mice. Survival is monitored daily, and moribund mice are humanely killed and considered deceased at time of death. Treatment with vehicle (5% dimethyl acetamide, 0.5% methocellulose) or Ruxolitinib (INCB018424) begins within 24 hours of cell inoculation, twice daily by oral gavage. Hematologic parameters are measured using a Bayer Advia120 analyzed, and statistical significance is determined using Dunnett testing. |
| References |
| Molecular Formula | C17H21N6O4P |
|---|---|
| Molecular Weight | 404.360 |
| Exact Mass | 404.136200 |
| PSA | 170.75000 |
| LogP | 2.53778 |
| Storage condition | -20℃ |
| Hazard Codes | N |
|---|---|
| HS Code | 2933990090 |
|
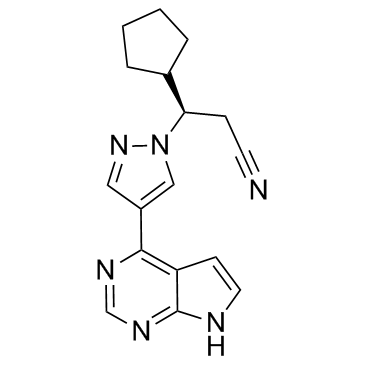
~81% 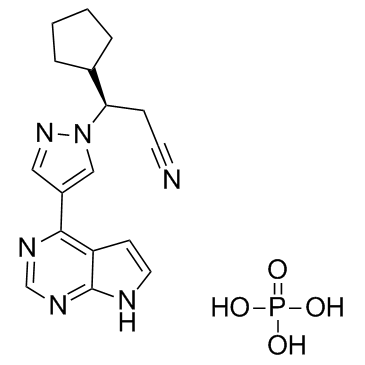
1092939-17-7 |
| Literature: US2010/190981 A1, ; Page/Page column 80 ; US 20100190981 A1 |
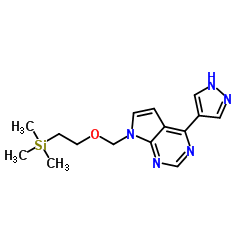
|
~% 
1092939-17-7 |
| Literature: WO2013/23119 A1, ; |
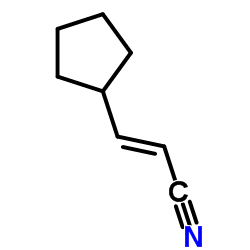
|
~% 
1092939-17-7 |
| Literature: WO2013/23119 A1, ; |
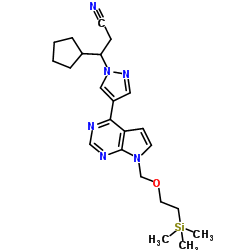
|
~% 
1092939-17-7 |
| Literature: WO2013/23119 A1, ; |
|
~% 
1092939-17-7 |
| Literature: WO2013/23119 A1, ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |