10212-30-3
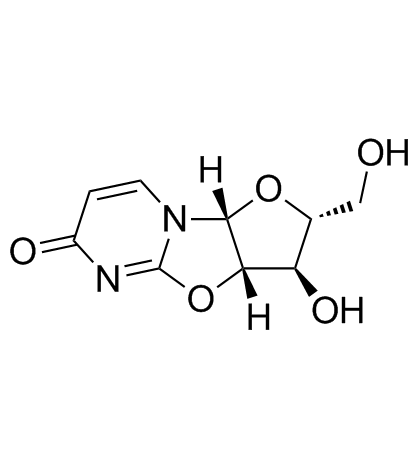
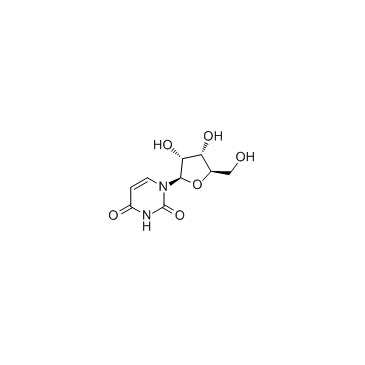
| Name | 2-amino-1-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-4-one |
|---|
| Description | 2-Amino-1-β-D-arabinofuranosyl-4(1H)-pyrimidinone is a purine nucleoside analogue. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.89g/cm3 |
|---|---|
| Boiling Point | 545.7ºC at 760mmHg |
| Molecular Formula | C9H13N3O5 |
| Molecular Weight | 243.22 |
| Flash Point | 283.8ºC |
| Exact Mass | 243.08600 |
| PSA | 131.82000 |
| Vapour Pressure | 3.5E-14mmHg at 25°C |
| Index of Refraction | 1.756 |
|
~% 
10212-30-3 |
| Literature: Ozaki, Hiroaki; Nakajima, Kiyohiro; Tatsui, Kaoru; Izumi, Chieko; Kuwahara, Masayasu; Sawai, Hiroaki Bioorganic and Medicinal Chemistry Letters, 2003 , vol. 13, # 15 p. 2441 - 2443 |
|
~% 
10212-30-3 |
| Literature: Brown et al. Journal of the Chemical Society, 1956 , p. 2388,2392 Journal of the Chemical Society, 1958 , p. 3028,3032 |
|
~% 
10212-30-3 |
| Literature: Brown et al. Journal of the Chemical Society, 1958 , p. 3028,3033 Full Text Show Details Brown et al. Journal of the Chemical Society, 1956 , p. 2388,2392 Journal of the Chemical Society, 1958 , p. 3028,3032 |
|
~% 
10212-30-3 |
| Literature: Brown et al. Journal of the Chemical Society, 1956 , p. 2388,2392 Journal of the Chemical Society, 1958 , p. 3028,3032 |
|
~% 
10212-30-3 |
| Literature: Brown et al. Journal of the Chemical Society, 1956 , p. 2388,2392 Journal of the Chemical Society, 1958 , p. 3028,3032 |
|
~% 
10212-30-3 |
| Literature: Brown et al. Journal of the Chemical Society, 1956 , p. 2388,2392 Journal of the Chemical Society, 1958 , p. 3028,3032 |
|
~% 
10212-30-3 |
| Literature: Brown et al. Journal of the Chemical Society, 1956 , p. 2388,2392 Journal of the Chemical Society, 1958 , p. 3028,3032 |
|
~% 
10212-30-3 |
| Literature: Brown et al. Journal of the Chemical Society, 1958 , p. 3028,3033 |
|
~% 
10212-30-3 |
| Literature: Brown et al. Journal of the Chemical Society, 1958 , p. 3028,3033 |
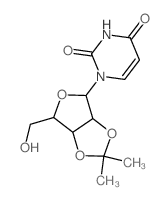
| Precursor 7 | |
|---|---|
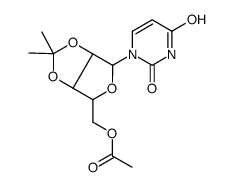
| DownStream 1 | |