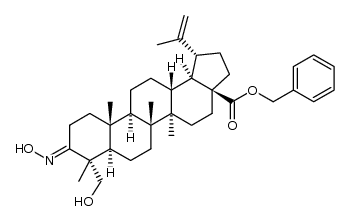
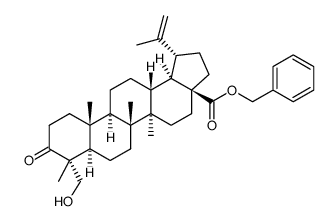
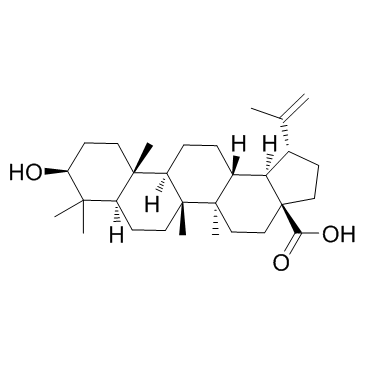
85999-40-2
| Name | (1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aS,13bR)-9-hydroxy-8-(hydroxymethyl)-5a,5b,8,11a-tetramethyl-1-prop-1-en-2-yl-1,2,3,4,5,6,7,7a,9,10,11,11b,12,13,13a,13b-hexadecahydrocyclopenta[a]chrysene-3a-carboxylic acid |
|---|---|
| Synonyms |
(3β,13α)-3,23-Dihydroxylup-20(29)-en-28-oic acid
Lup-20(29)-en-28-oic acid, 3,23-dihydroxy-, (3β,13α)- Anemosapogenin (3β)-3,23-Dihydroxylup-20(29)-en-28-oic acid 23-Hydroxybetulinic acid 3|A,23-Dihydroxylup-20(29)-en-28-oic acid Lup-20(29)-en-28-oic acid, 3,23-dihydroxy-, (3β)- 23-hydroxy butulinic acid 23-HBA |
| Description | 23-hydroxybetulinic acid is one of the bioactive components responsible for its anticancer activity.In vitro: 23-hydroxybetulinic acid also shows different proliferation inhibitory activity against B16, HeLa, and HUVEC, with the IC50 value of 78.5, 80, and 94.8 uM, respectively. 23-hydroxybetulinic acid can promote cell cycle arrest at S phase and induce apoptosis via intrinsic pathway. 23-hydroxybetulinic acid disrupts mitochondrial membrane potential significantly (p<0.01) and selectively downregulates the levels of Bcl-2, survivin and upregulates Bax, cytochrome C, cleaved caspase-9 23-hydroxybetulinic acid can induce apoptosis in K562 cells. [1] 23-hydroxybetulinic acid enhances sensitivity of doxorubicin (DOX, ADR) on MCF-7/ADR cell lines, indicating its potential to be developed as a novel MDR modulator.[2] 23-HBA significantly improve the sensitivity of the tumor to doxorubicin. [3] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 585.8±25.0 °C at 760 mmHg |
| Melting Point | 305 °C |
| Molecular Formula | C30H48O4 |
| Molecular Weight | 472.700 |
| Flash Point | 322.1±19.7 °C |
| Exact Mass | 472.355255 |
| PSA | 77.76000 |
| LogP | 7.29 |
| Vapour Pressure | 0.0±3.7 mmHg at 25°C |
| Index of Refraction | 1.543 |
|
~86% 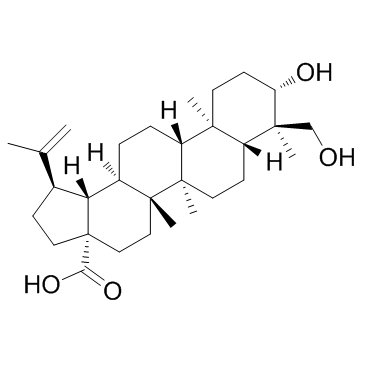
85999-40-2 |
| Literature: Sun, Fei; Zhu, Peiqing; Yao, Hequan; Wu, Xiaoming; Xu, Jinyi Journal of Chemical Research, 2012 , vol. 36, # 5 p. 254 - 257 |
|
~% 
85999-40-2 |
| Literature: Journal of Chemical Research, , vol. 36, # 5 p. 254 - 257 |
|
~% 
85999-40-2 |
| Literature: Journal of Chemical Research, , vol. 36, # 5 p. 254 - 257 |
|
~% 
85999-40-2 |
| Literature: Journal of Chemical Research, , vol. 36, # 5 p. 254 - 257 |
|
~% 
85999-40-2 |
| Literature: Journal of Chemical Research, , vol. 36, # 5 p. 254 - 257 |
|
~% 
85999-40-2 |
| Literature: Journal of Chemical Research, , vol. 36, # 5 p. 254 - 257 |
|
~% 
85999-40-2 |
| Literature: Journal of Chemical Research, , vol. 36, # 5 p. 254 - 257 |
|
~% 
85999-40-2 |
| Literature: Journal of Chemical Research, , vol. 36, # 5 p. 254 - 257 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |