202138-50-9
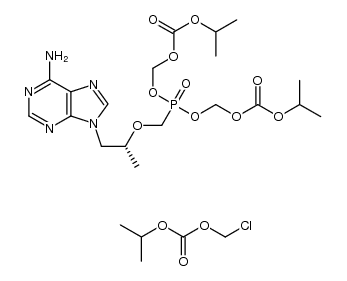
| Name | tenofovir disoproxil fumarate |
|---|---|
| Synonyms |
GS-4331-05
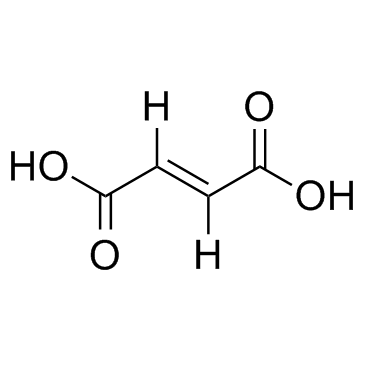
Viread (TN) Tenofovir diisoproxil fuMarate Carbonic acid, [[[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl][[[(1-methylethoxy)carbonyl]oxy]methoxy]phosphinyl]oxy]methyl 1-methylethyl ester, (2E)-2-butenedioate (1:1) Tenofovir disoproxil fumarate Bis(((isopropoxycarbonyl)oxy)methyl) (((1R)-2-(6-Amino-9H-purin-9-yl)-1-methylethoxy)methyl)phosphonate (2E)-2-Butenedioate acide (2E)-but-2-ènedioïque - {[(1R)-2-(6-amino-9H-purin-9-yl)-1-méthyléthoxy]méthyl}phosphonate de bis({[(1-méthyléthoxy)carbonyl]oxy}méthyle) (1:1) Bis{[(isopropoxycarbonyl)oxy]methyl} ({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy}methyl)phosphonate (2E)-but-2-enedioate (1:1) bis({[(propan-2-yloxy)carbonyl]oxy}methyl) ({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy}methyl)phosphonate (2E)-but-2-enedioate (1:1) Viread bis({[(1-methylethoxy)carbonyl]oxy}methyl) {[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}phosphonate (2E)-but-2-enedioate Bis{[(isopropoxycarbonyl)oxy]methyl} ({[(2R)-1-(6-amino-9H-purin-9-yl)-2-propanyl]oxy}methyl)phosphonate (2E)-2-butenedioate (1:1) TENOFOVIR DISOPROXIC Tenofovir DF 9-((R)-2-((Bis(((isopropoxycarbonyl)oxy)methoxy)phosphinyl)methoxy)propyl)adenine Fumarate Tenofovir (Disoproxil Fumarate) |
| Description | Tenofovir Disoproxil Fumarate is a nucleotide reverse transcriptase inhibitor used to treat HIV and chronic Hepatitis B. |
|---|---|
| Related Catalog | |
| In Vitro | Tenofovir shows cytotoxic effects on cell viability in HK-2 cells, with IC50 values of 9.21 and 2.77 μM at 48 and 72 h in MTT assay, respectively. Tenofovir diminishes ATP levels in HK-2 cells. Tenofovir (3.0 to 28.8 μM) increases oxidative stress and protein carbonylation in HK-2 cells. Furthermore, Tenofovir induces apoptosis in HK-2 cells, and that apoptosis is induced via mitochondrial damage[1]. Tenofovir and M48U1 formulated in 0.25% HEC each inhibits the replication of both R5-tropic HIV-1BaL and X4-tropic HIV-1IIIb in activated PBMCs, and inhibits several laboratory strains and patient-derived HIV-1 isolates. The combined formulation of M48U1 and tenofovir in 0.25% HEC exhibits synergistic antiretroviral activity against infection with R5-tropic HIV-1BaL, and is not toxic to PBMCs[2]. |
| In Vivo | Tenofovir Disoproxil Fumarate (20, 50, 140, or 300 mg/kg) administered to BLT mice, shows dose dependent activity during vaginal HIV challenge in BLT humanized mice. Tenofovir Disoproxil Fumarate (50, 140, 300 mg/kg) significantly reduces HIV transmission in BLT mice[3]. Tenofovir Disoproxil Fumarate (0.5, 1.5, or 5.0 mg/kg/day, p.o.) induces a dose-dependent decline in serum viremia in woodchucks chronically infected with WHV. Tenofovir Disoproxil Fumarate administration is safe and effective in the woodchuck model of chronic HBV infection[4]. |
| Cell Assay | Cells are plated into 48-well tissue culture plates (39,000 cells/mL) and allowed to grow for 48 h followed by treatment with vehicle or Tenofovir. Following the treatment period, cell viability is assessed using the MTT assay. The MTT assay relies on the conversion of tetrazolium dye 3-(4,5-dimethlthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan by NAD(P)H-dependent oxidoreductases. |
| Animal Admin | Twenty adult chronic WHV carrier woodchucks are stratified equally by age, sex, body weight, and serum GGT activity into five treatment groups consisting of four animals each: (i) Tenofovir Disoproxil Fumarate at 15.0 mg/kg once per day, (ii) Tenofovir Disoproxil Fumarate at 5.0 mg/kg/day, (iii) Tenofovir Disoproxil Fumarate at 1.5 mg/kg/day, (iv) Tenofovir Disoproxil Fumarate at 0.5 mg/kg/day, and (v) a placebo control. The woodchucks are treated daily for 4 weeks and observed for an additional 12 weeks following cessation of drug treatment. |
| References |
| Density | 1.45 g/cm3 |
|---|---|
| Boiling Point | 642.7ºC at 760 mmHg |
| Melting Point | 219ºC |
| Molecular Formula | C23H34N5O14P |
| Molecular Weight | 635.515 |
| Flash Point | 342.5ºC |
| Exact Mass | 635.183960 |
| PSA | 269.85000 |
| LogP | 3.32860 |
| Vapour Pressure | 2.06E-16mmHg at 25°C |
| Storage condition | Refrigerator |
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H318 |
| Precautionary Statements | P280-P305 + P351 + P338 + P310 |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
|
~% 
202138-50-9 |
| Literature: US2013/5969 A1, ; Page/Page column 4 ; |
|
~% 
202138-50-9 |
| Literature: WO2011/111074 A2, ; Page/Page column 8; 9 ; |
|
~% 
202138-50-9 |
| Literature: Organic Process Research and Development, , vol. 14, # 5 p. 1194 - 1201 |
|
~% 
202138-50-9 |
| Literature: Organic Process Research and Development, , vol. 14, # 5 p. 1194 - 1201 |
|
~% 
202138-50-9 |
| Literature: Organic Process Research and Development, , vol. 14, # 5 p. 1194 - 1201 |
|
~% 
202138-50-9 |
| Literature: Organic Process Research and Development, , vol. 14, # 5 p. 1194 - 1201 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |