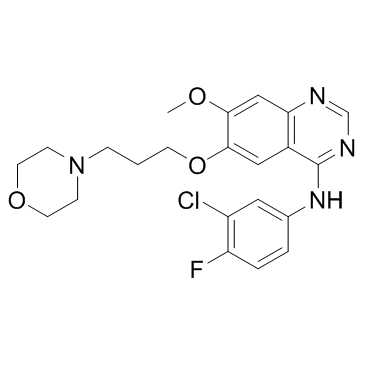
184475-35-2
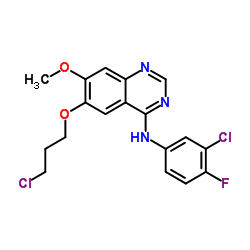
| Name | gefitinib |
|---|---|
| Synonyms |
Iressa
N-(3-Chloro-4-fluorophenyl)-7-methoxy-6-[3-(4-morpholinyl)propoxy]-4-quinazolinamine N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-(4-morpholinyl)propoxy)-4-quinazolinamide MFCD04307832 Irressat N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(morpholin-4-yl)propoxy]quinazolin-4-amine N-(3-chloro-4-fluoro-phenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine N-(3-Chlor-4-fluorphenyl)-7-methoxy-6-[3-(morpholin-4-yl)propoxy]chinazolin-4-amin N-(3-Chlor-4-fluorphenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)chinazolin-4-amin N-(3-Chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-amine N-(3-chloro-4-fluorophényl)-7-méthoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine ZD-1839 Gefitinib 4-Quinazolinamine, N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(4-morpholinyl)propoxy]- N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine ZD1839 |
| Description | Gefitinib (ZD1839) is a EGFR tyrosine kinase inhibitor, with IC50 of 2-37 nM in NR6wtEGFR cells. |
|---|---|
| Related Catalog | |
| Target |
EGFR |
| In Vitro | Gefitinib (0.01-0.1 mM) results in increased phosphotyrosine load of the receptor, increased signalling to ERK and stimulation of proliferation and anchorage-independent growth, presumably by inducing EGFRvIII dimerisation in long-term exposure of EGFRvIII-expressing cells. On the other hand, gefitinib (1-2 mM) significantly decreases EGFRvIII phosphotyrosine load, EGFRvIII-mediated proliferation and anchorage-independent growth[1]. Gefitinib (ZD1839) inhibits the monolayer growth of these EGF-driven untransformed cells with IC50 of 20 nM[2]. Gefitinib leads to an inhibition of CALU-3 and GLC82 cell proliferation, with an IC50 of 2 μM[3]. |
| In Vivo | Gefitinib (150 mg/kg, p.o.) in conbination with Metformin induces a significant reduction in tumor growth in nude mice bearing H1299 or CALU-3 GEF-R cells that are grown subcutaneously as tumor xenografts[3]. In irradiated rats, Gefitinib treatment augmentes lung inflammation, including inflammatory cell infiltration and pro-inflammatory cytokine expression, while Gefitinib treatment attenuates fibrotic lung remodeling due to the inhibition of lung fibroblast proliferation[4]. |
| Cell Assay | Cancer cells are seeded in 96-well plates and are treated with different doses of Gefitinib (0.01-20 μM), Metformin or both for 72 hours. Cell proliferation is measured with the MTT assay. The IC50 values are determined by interpolation from the dose-response curves. Results represent the median of 3 separate experiments each conducted in quadruplicate. The results of the combined treatment are analyzed according to the method of Chou and Talalay by using the CalcuSyn software program[3]. |
| Animal Admin | Mice[3] Four- to 6-week old female balb/c athymic (nu+/nu+) mice are acclimatized for 1 week before being injected with cancer cells and injected subcutaneously with 107 H1299 and CALU-3 GEF-R cells that has been resuspended in 200 μL of Matrigel. When established tumors of approximately 75 mm3 in diameter are detected, mice are left untreated or treated with oral administrations of metformin (200 mg/mL metformin diluted in drinking water and present throughout the experiment), gefitinib (150 mg/kg daily orally by gavage), or both for the indicated time periods. Each treatment group consists of 10 mice. Tumor volume is measured using the formula π/6×larger diameter×(smaller diameter)2. Rats[4] The rats are randomly assigned to 1 of 4 experimental groups: 1) the unirradiated rats treated with oral administration of vehicle (0.1% Tween 80) once daily; 2) the unirradiated rats treated with oral administration of gefitinib (50 mg/kg/day) once daily; 3) the irradiated rats treated with oral administration of vehicle once daily; 4) the irradiated rats treated with oral administration of gefitinib once daily. Each experimental group comprised 5-6 rats and all treatments are delivered for 14 days. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 586.8±50.0 °C at 760 mmHg |
| Melting Point | 119-1200C |
| Molecular Formula | C22H24ClFN4O3 |
| Molecular Weight | 446.902 |
| Flash Point | 308.7±30.1 °C |
| Exact Mass | 446.152100 |
| PSA | 68.74000 |
| LogP | 4.11 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.621 |
| Storage condition | Store at RT |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P301 + P312 + P330-P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | HP1149700 |
| HS Code | 29143900 |
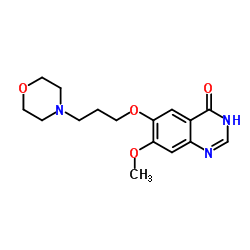
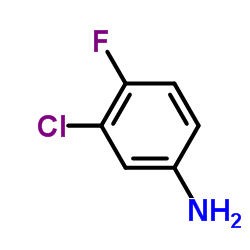
| Precursor 7 | |
|---|---|
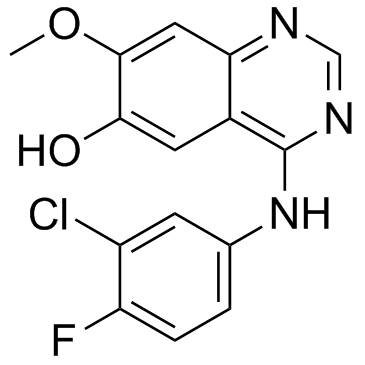
| DownStream 2 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |