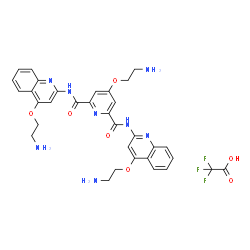
1472611-44-1
| Name | Pyridostatin TFA salt |
|---|---|
| Synonyms |
4-(2-Aminoethoxy)-N,N'-bis[4-(2-aminoethoxy)-2-quinolinyl]-2,6-pyridinedicarboxamide trifluoroacetate (1:1)
RR82 trifluoroacetate salt 4-(2-Aminoethoxy)-N2,N6-bis[4-(2-aminoethoxy)-2-quinolinyl]-2,6-pyridinedicarboxamide trifluoroacetate salt Acetic acid, 2,2,2-trifluoro-, compd. with 4-(2-aminoethoxy)-N2,N6-bis[4-(2-aminoethoxy)-2-quinolinyl]-2,6-pyridinedicarboxamide (1:1) Pyridostatin Trifluoroacetate Salt MFCD26142941 |
| Description | Pyridostatin (RR82) TFA is a G-quadruplex DNA stabilizing agent (Kd=490 nM). Pyridostatin TFA promotes growth arrest in human cancer cells by inducing replication- and transcription-dependent DNA damage. Pyridostatin TFA targets the proto-oncogene Src. Pyridostatin TFA reduced SRC protein levels and SRC-dependent cellular motility in human breast cancer cells[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Pyridostatin (RR82) hydrochloride (10 μM; 48 hours) induces cell cycle arrest[1]. Pyridostatin TFA is a very selective G-quadruplex DNA-binding small molecule designed to form a complex with and stabilize G-quadruplex structure. Pyridostatin TFA causes neurite retraction, synaptic loss, and dose-dependent neuronal death. In cultured primary neurons, Pyridostatin TFA induces the formation of DNA DSBs. Remarkably, Pyridostatin TFA (1-5 μM, overnight) downregulates the BRCA1 protein, a protein that guards and repairs the neuronal genome, at the transcriptional level[3]. Cell Viability Assay[1] Cell Line: Over 60 different cancer cell lines Concentration: 10 μM Incubation Time: 48 hours Result: Predominantly accumulated in the G2 phase of the cell cycle over 60 different cancer cell lines. |
| References |
| Molecular Formula | C33H33F3N8O7 |
|---|---|
| Molecular Weight | 710.660 |
| Exact Mass | 710.242432 |