40951-21-1
| Name | 2-Hydroxyglutaric Acid Disodium Salt |
|---|---|
| Synonyms |
hydroxyglutaric acid
Pentanedioic acid, 2-hydroxy- 2-Hydroxypentanedioic acid 2-hydroxyglutaric acid DL-2-Hydroxyglutaric (RS)-2-Hydroxypentanedioic acid disodium salt Disodium 2-hydroxyglutarate 2-Hydroxy-pentanedioic acid |
| Description | α-Hydroxyglutaric acid (2-Hydroxyglutarate) disodium is an α-hydroxy acid form of glutaric acid. α-Hydroxyglutaric acid disodium is a competitive inhibitor of multiple α-ketoglutarate-dependent dioxygenases, including histone demethylases and the TET family of 5-methlycytosine (5mC) hydroxylases[1]. |
|---|---|
| Related Catalog | |
| Target |
Microbial Metabolite Human Endogenous Metabolite |
| In Vitro | Isocitrate Dehydrogenase 1 (IDH1) and IDH2 mutations occur frequently in gliomas and acute myeloid leukemia, leading to simultaneous loss and gain of activities in the production of α-ketoglutarate (α-KG) and α-Hydroxyglutaric acid (2-Hydroxyglutarate) disodium, respectively[1]. α-Hydroxyglutaric acid (2-Hydroxyglutarate) disodium inhibits the activity of multiple histone demethylases. α-Hydroxyglutaric acid occupies the same space as α-KG does in the active site of histone demethylases. α-Hydroxyglutaric acid (2-Hydroxyglutarate) disodium inhibits the activity of TET 5-methylcytosine hydroxylases[1]. Treatment of U-87MG cells with α-Hydroxyglutaric acid (2-Hydroxyglutarate; 10-50 mM) disodium increases HIF-1α and decreases endostatin[1]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 394.2±27.0 °C at 760 mmHg |
| Melting Point | >290ºC (dec.) |
| Molecular Formula | C5H8O5 |
| Molecular Weight | 148.114 |
| Flash Point | 206.4±20.2 °C |
| Exact Mass | 148.037170 |
| PSA | 94.83000 |
| LogP | -1.45 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.520 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~63% 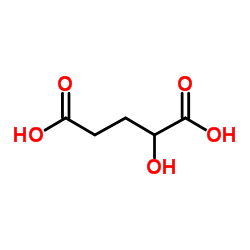
40951-21-1 |
| Literature: Heizmann, Gerhard; Pfleiderer, Wolfgang Helvetica Chimica Acta, 2007 , vol. 90, # 10 p. 1856 - 1873 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |