669073-68-1
| Name | ZK216348 |
|---|---|
| Synonyms |
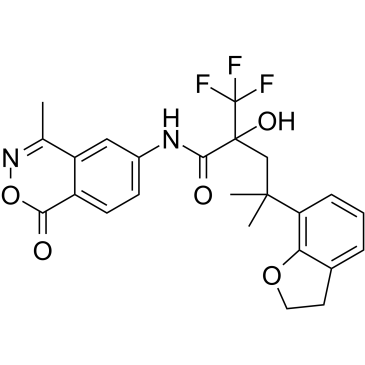
7-Benzofuranbutanamide, 2,3-dihydro-α-hydroxy-γ,γ-dimethyl-N-(4-methyl-1-oxo-1H-2,3-benzoxazin-6-yl)-α-(trifluoromethyl)-
8BY7XK862L ZK216348 4-(2,3-Dihydro-1-benzofuran-7-yl)-2-hydroxy-4-methyl-N-(4-methyl-1-oxo-1H-2,3-benzoxazin-6-yl)-2-(trifluoromethyl)pentanamide |
| Description | ZK 216348 ((+)-ZK 216348) is a nonsteroidal selective glucocorticoid receptor agonist with an IC50 of 20.3 nM. ZK 216348 also binds to Progesterone and mineralocorticoid receptors with IC50s of 20.4 nM and 79.9 nM, respectively. ZK 216348 has antiinflammatory activity similar to Prednisolone and induces less transactivation-mediated side effects[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 20.3 nM (Glucocorticoid recepto), 20.4 nM (Progesterone receptor ) and 79.9 nM (mineralocorticoid receptor)[1] |
| In Vitro | In human peripheral blood mononuclear cells (PBMCs), ZK 216348 inhibits TNF-α and IL-12 with IC50 of 89 nM and 52 nM, respectively[1]. Participation of an active GR in the antiinflammatory response of ZK 216348 is further investigated in Caco-2 cells, where the TNF-α-induced expression of the proinflammatory cytokine IL-8 is suppressed in the presence of ZK 216348[2]. |
| In Vivo | ZK 216348 (1-30 mg/kg; subcutaneous injection; for 24 hours; NMRI mice and Wistar rats) treatment inhibits ear edema in both mice and rats. A markedly superior side-effect profile is found in ZK 216348 with regard to increases in blood glucose, spleen involution, and, to a lesser extent, skin atrophy[1]. Animal Model: NMRI mice (26-28 g) and Wistar rats (140-160 g) injection with Croton oil[1] Dosage: 1 mg/kg, 3 mg/kg, 10 mg/kg and 30 mg/kg Administration: Subcutaneous injection; for 24 hours Result: Inhibited ear edema in mice and rats. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C24H23F3N2O5 |
| Molecular Weight | 476.445 |
| Exact Mass | 476.155914 |
| LogP | 4.39 |
| Index of Refraction | 1.590 |