49562-28-9
| Name | fenofibrate |
|---|---|
| Synonyms |
propan-2-yl 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoate
Lipantil Isopropyl 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionate Procetofen 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic Acid Isopropyl Ester Fenofibrate Lipidil Tricor Isopropyl 2-(4-(4-chlorobenzoyl)phenoxy)-2-methylpropanoate 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropanoic acid isopropyl ester Lipanthyl Supralip Secalip |
| Description | Fenofibrate is a PPARα agonist with an EC50 of 30 μM. |
|---|---|
| Related Catalog | |
| Target |
CYP2C19:0.2 μM (IC50) CYP2B6:0.7 μM (IC50) CYP2C9:9.7 μM (IC50) CYP2C8:4.8 μM (IC50) CYP3A4:142.1 μM (IC50) PPARα:30 μM (IC50) |
| In Vitro | Fenofibrate is a relatively potent inhibitor of CYP2B6 (IC50=0.7±0.2 μM) and CYP2C19 (IC50=0.2±0.1 μM). Fenofibrate is also a moderate inhibitor of CYP2C8 (IC50=4.8±1.7 μM) and CYP2C9 (IC50=9.7 μM)[1]. Fenofibrate binds to and inhibits cytochrome P450 epoxygenase (CYP)2C with higher affinity than to PPARα. Fenofibrate is a well-known PPARα agonist, but an in vitro assessment of 209 frequently prescribed drugs and related xenobiotics suggests that Fenofibrate is also a potent inhibitor of cytochrome P450 epoxygenase (CYP)2C. The affinity of Fenofibrate to CYP2C is >10 times higher (EC50=2.39±0.4 μM) than to PPARα (EC50=30 μM). Fenofibrate at a low dose inhibits CYP2C8 activity without PPARα activation[2]. |
| In Vivo | Daily intake of Fenofibrate at this low dose (10 μg/g/day) inhibits retinal and choroidal neovascularization induced by CYP2C8 overexpression by 29% (P=0.021) and 36% (P=1.2×10−9) respectively[2]. |
| Kinase Assay | The half-maximal inhibitory concentrations (IC50s) of Fenofibrate, statins (atorvastatin, lovastatin, pravastatin, simvastatin and simvastatin acid, the active form of simvastatin) and glipizide for recombinant human CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 are determined using fluorometric CYP450 inhibition assays. Briefly, the drugs are dissolved in methanol or acetonitrile. In 96 well assay plates, the drugs are diluted to a series of concentrations in a solution containing cofactors including NADP+ (final concentration 1.3 mM), MgCl2 (final concentration 3.3 m M), glucose-6-phosphate (G6P, final concentration 3.3 mM) and glucose 6-phosphate dehydrogenase (final concentration 0.4 U/mL). The mixture is pre-incubated at 37°C for 10 min. The enzymes and fluorogenic substrates are diluted to desired concentrations in sodium phosphate reaction buffer (pH 7.4, final concentration 200 mM) and mixed. Reactions are initiated with addition of the enzyme and substrate mixture to the cofactor and drug mixture. The final reaction volume of all assays is 200 μL. After incubating at 37°C for a pre-specified period of time (15 to 45 min), the reactions are stopped with addition of 75 μL quenching solution (0.5 M Tris base or 2N NaOH). Fluorescence is determined using a BioTek Synergy 2 fluorescence reader. Each of the drugs is tested at eight concentrations in duplicate. To estimate IC50s, percent of inhibition is calculated using net fluorescence that is corrected for the background. The values of percent of inhibition are then fitted to a three or four parameter log-logistic model[1]. |
| Animal Admin | Mice[2] The mouse oxygen-induced retinopathy (OIR) model is used. Briefly to induce retinal neovascularization, mouse pups and their nursing mother are exposed to 75±3% oxygen from P7 to P12. For the higher dose Fenofibrate (F6020) treatment (100 mg/kg/day). Fenofibrate is dissolved in corn oil to make 100mg/mL solution and pure corn oil is used as vehicle control. For the lower dose treatment (10 mg/kg/day), Fenofibrate is dissolved in 10% DMSO, D2650 to make a 10 mg/mL solution and 10% DMSO is used as vehicle control. After return to room air, mice are orally gavaged with Fenofibrate (100 or 10 mg/kg) or vehicle control daily from P12 to P16. At P17, eyes are enucleated immediately after euthanasia and fixed in 4% paraformaldehyde in PBS for 1 h at room temperature. Retinas are then dissected and stained overnight with Alexa Fluor 594 conjugated isolectin GS-IB4 (10 μg/mL) at room temperature. After washing with PBS, retinas are mounted onto microscope slides with photoreceptor side down and embedded in SlowFade antifade mounting medium. Retinal images are taken using a fluorescence microscope with image software. Retinal neovascularization is analyzed. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 469.8±35.0 °C at 760 mmHg |
| Melting Point | 80-81ºC |
| Molecular Formula | C20H21ClO4 |
| Molecular Weight | 360.831 |
| Flash Point | 165.4±24.9 °C |
| Exact Mass | 360.112823 |
| PSA | 52.60000 |
| LogP | 4.80 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.547 |
| Storage condition | Store at RT |
| Water Solubility | Practically insoluble in water, very soluble in methylene chloride, slightly soluble in ethanol (96 per cent) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UA2453400 |
| HS Code | 2932999099 |
|
~94% 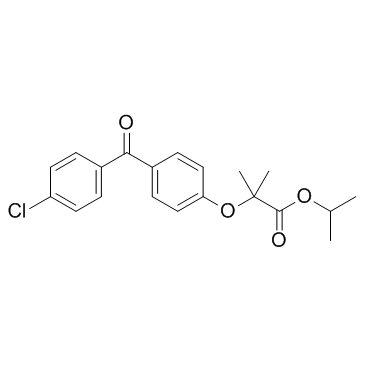
49562-28-9 |
| Literature: US2010/185008 A1, ; Page/Page column 2 ; |
|
~98% 
49562-28-9 |
| Literature: US2012/65421 A1, ; Page/Page column 2-3 ; |
|
~% 
49562-28-9 |
| Literature: FR2300552DE2605382 , ; Chem.Abstr., , vol. 85, # 192382 |
|
~83% 
49562-28-9 |
| Literature: Journal of Organic Chemistry, , vol. 78, # 20 p. 10310 - 10318 |
|
~% 
49562-28-9 |
| Literature: EP1837327 A1, ; Page/Page column 4 ; |
|
~% 
49562-28-9 |
| Literature: FR2300552DE2605382 , ; Chem.Abstr., , vol. 85, # 192382 |
|
~% 
49562-28-9 |
| Literature: FR2300552DE2605382 , ; Chem.Abstr., , vol. 85, # 192382 |
|
~%
Detail
|
| Literature: Arzneimittel-Forschung, , vol. 29, # 5 p. 711 - 720 |
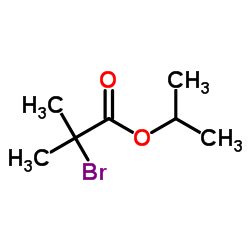
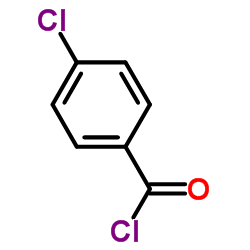
| Precursor 10 | |
|---|---|
| DownStream 2 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |






![propan-2-yl 2-[4-[(4-chlorophenyl)-hydroxymethyl]phenoxy]-2-methylpropanoate structure](https://image.chemsrc.com/caspic/398/61001-99-8.png)