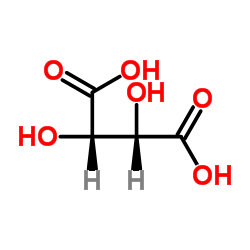
209394-46-7
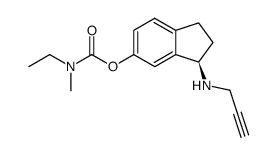
| Name | (2R,3R)-2,3-dihydroxybutanedioic acid,[(3R)-3-(prop-2-ynylamino)-2,3-dihydro-1H-inden-5-yl] N-ethyl-N-methylcarbamate |
|---|---|
| Synonyms |
ethyl-methyl-carbamic acid (R)-3-prop-2-ynylamino-indan-5-yl ester tartrate
TV-3326 Ladostigil tartrate (3R)-3-(prop-2-ynylamino)-2,3,-dihydro-1H-indan-5-yl ethyl methyl carbamate tartrate [(3R)-3-(prop-2-ynylamino)-2,3-dihydro-1H-inden-5-yl] N-ethyl-N-methylcarbamate R(+)-6-(N-methyl-N-ethylcarbamoyloxy)-N'-propargyl-1-aminoindan tartrate UNII-2J1346C51H (2R,3R)-2,3-dihydroxybutanedioic acid ethylmethylcarbamic acid (3R)-2,3-dihydro-3-(2-propynylamino)-1H-inden-5-yl ester (2R,3R)-2,3-dihydroxybutanedioate R(+)-6-(N-methyl-N-ethylcarbamoyloxy)-N'-propargyl-1-aminoindan L-tartrate TV-3219 (2R,3R)-2,3-Dihydroxysuccinic acid - (3R)-3-(2-propyn-1-ylamino)-2,3-dihydro-1H-inden-5-yl ethyl(methyl)carbamate (1:2) |
| Description | Ladostigil (TV-3326) hemitartrate is a dual inhibitor of cholinesterase and brain-selective monoamine oxidase (MAO), with an IC50 of 37.1 and 31.8 μM for MAO-B and AChE, reapectively. Ladostigil hemitartrate could increase cholinergic transmission, prevent the formation of ROS or their actions and be used for the research of depression and Alzheimer's disease[1][2]. |
|---|---|
| Related Catalog | |
| Target |
MAO-B:37.1 nM (IC50) AChE:31.8 nM (IC50) |
| In Vitro | Ladostigil (1-10 µM) hemitartrate exerts neuroprotective activities, including a prevention of the fall of the mitochondrial membrane potential (ψ), attenuation of apoptotic cascades and an inhibition of ROS production induced by OS insults[2]. Ladostigil (1-10 µM) hemitartrate has a significant neuroprotective activity, including inhibition of caspase-3 activation, induction of Bcl-2 and reduction of Bad and Bax gene and protein expression in human neuroblastoma SK-N-SH cells[2]. |
| In Vivo | Ladostigil (17 mg/kg; p.o. daily for 6 weeks) hemitartrate abolishes their hyperanxiety and depressive-like behaviour in the elevated plus maze (EPM) and forced swim tests (FST) tests in adulthood from puberty to prenatally-stressed rats[4]. Ladostigil (50 μmol/kg; single p.o.) hemitartrate restores the loss of episodic memory in the object recognition test in rats[3]. Animal Model: Pathogen-free (SPF) Sprague-Dawley rats[4] Dosage: 17 mg/kg Administration: P.o. (added to the drinking water) daily for 6 weeks Result: Inhibited brain MAO-A and B by more than 60%. Reduced hyperanxiety of male and female prenatally stressed (PS) rats in the EPM and depressive-like behaviour in the FST. |
| References |
| Boiling Point | 412.4ºC at 760 mmHg |
|---|---|
| Molecular Formula | C16H20N2O2.1/2C4H6O6 |
| Molecular Weight | 694.77 |
| Flash Point | 203.2ºC |
| Exact Mass | 694.321411 |
| PSA | 198.20000 |
| LogP | 3.35360 |
| Vapour Pressure | 5.19E-07mmHg at 25°C |
| Hazard Codes | Xi |
|---|
|
~87% 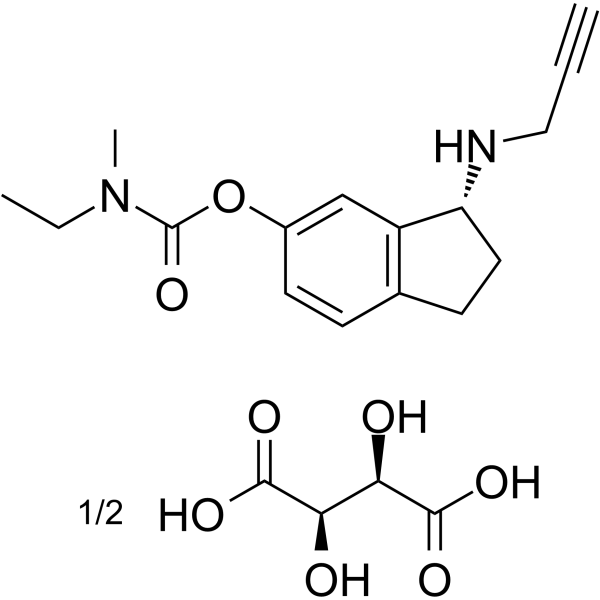
209394-46-7 |
| Literature: Aronhime, Judith; Bahar, Eliezer; Frenkel, Anton; Gottesfeld, Ronen; Gold, Amir; Koltai, Tamas Patent: US2007/88082 A1, 2007 ; Location in patent: Page/Page column 13 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |