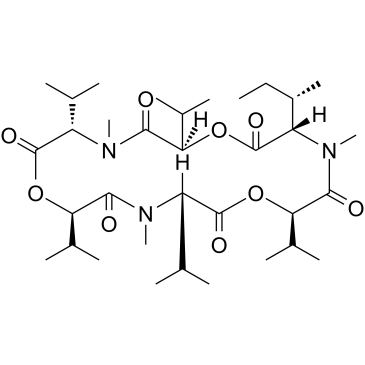
19914-20-6
| Name | Enniatin B1 |
|---|---|
| Synonyms |
1,7,13-Trioxa-4,10,16-triazacyclooctadecane-2,5,8,11,14,17-hexone, 4,10,16-trimethyl-3,6,9,12,18-pentakis(1-methylethyl)-15-[(1R)-1-methylpropyl]-, (3S,6R,9S,12R,15R,18R)-
1,7,13-Trioxa-4,10,16-triazacyclooctadecane-2,5,8,11,14,17-hexone, 4,10,16-trimethyl-3,6,9,12,18-pentakis(1-methylethyl)-15-[(1S)-1-methylpropyl]-, (3S,6R,9S,12R,15S,18R)- (3R,6R,9S,12R,15S,18R)-3-[(2R)-2-Butanyl]-6,9,12,15,18-pentaisopropyl-4,10,16-trimethyl-1,7,13-trioxa-4,10,16-triazacyclooctadecane-2,5,8,11,14,17-hexone (3S,6R,9S,12R,15S,18R)-3-[(2S)-2-Butanyl]-6,9,12,15,18-pentaisopropyl-4,10,16-trimethyl-1,7,13-trioxa-4,10,16-triazacyclooctadecane-2,5,8,11,14,17-hexone |
| Description | Enniatin B1 is a Fusarium mycotoxin. Enniatin B1 inhibits acyl-CoA: cholesterol acyltransferase (ACAT) activity with an IC50 of 73 μM in an enzyme assay using rat liver microsomes[1]. Enniatin B1 crosss the blood-brain barrier[2]. Enniatin B1 decreases the activation of ERK (p44/p42). Enniatin B1 inhibits moderately TNF-α-induced NF-κB activation[3]. |
|---|---|
| Related Catalog | |
| Target |
ACAT ERK NF-κB |
| In Vitro | CCF-STTG1 cells are sensitive to Enniatin B1 (IC50=4.4 μM)[2]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 833.8±65.0 °C at 760 mmHg |
| Molecular Formula | C34H59N3O9 |
| Molecular Weight | 653.847 |
| Flash Point | 458.0±34.3 °C |
| Exact Mass | 653.425110 |
| PSA | 139.83000 |
| LogP | 3.58 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.460 |
| Storage condition | ?20°C |
| Water Solubility | DMSO: soluble10mg/mL |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H311-H331 |
| Precautionary Statements | P261-P280-P301 + P310-P311 |
| Hazard Codes | T |
| Risk Phrases | 23/24/25 |
| Safety Phrases | 45 |
| RIDADR | 2811 |
| Packaging Group | I |