518-17-2
| Name | Evodiamine |
|---|---|
| Synonyms |
EVODIAMINE PLANT EXTRACT
Indolo[2',3':3,4]pyrido[2,1-b]quinazolin-5(7H)-one, 8,13,13b,14-tetrahydro-14-methyl-, (13bS)- Evodia rutaecarpa E.P. Indol(2',3':3,4)pyrido(2,1-b)quinazolin-5(7H)-one, 8,13,13b,14-tetrahydro-14-methyl-, (13bS)- Indol(2',3':3,4)pyrido(2,1-b)quinazolin-5(7H)-one, 8,13,13b,14-tetrahydro-14-methyl-, (S)- d-Evodiamine (13bS)-14-Methyl-8,13,13b,14-tetrahydroindolo[2',3':3,4]pyrido[2,1-b]quinazolin-5(7H)-one MFCD06407824 |
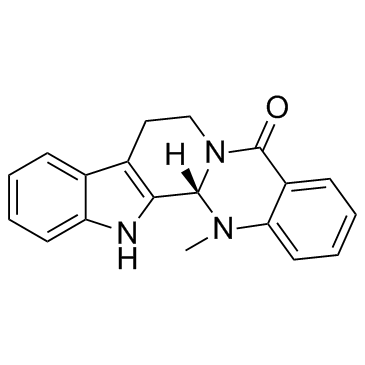
| Description | Evodiamine is an alkaloid isolated from the fruit of Evodia rutaecarpa Bentham with diverse biological activities including anti-inflammatory, anti-obesity, and antitumor. |
|---|---|
| Related Catalog | |
| In Vitro | Evodiamine shows cytotoxicity against a variety of human cancer cell-lines by inducing apoptosis. Moreover, it is a naturally multi-targeting antitumor molecule, which exerts the antitumor activity by various molecular mechanism such as caspase-dependent and -independent pathways, sphingomyelin pathway, calcium/JNK signaling, 31 PI3K/Akt/caspase and Fas-L/NF-κB signaling pathways32[1]. |
| In Vivo | Evodiamine inhibits the metabolism of dapoxetine. Compared to the control group, the pharmacokinetic parameter of t1/2, AUC(0-∞) and Tmax of dapoxetine in evodiamine group is significantly increased by 63.3%, 44.8% and 50.4%, respectively. Moreover, evodiamine has significantly decreased the pharmacokinetic parameter of t1/2 and AUC(0-∞) of desmethyl dapoxetine[2]. Evodiamine suppresses tumor growth in a subcutaneous H22 xenograft model. Evodiamine attenuates VEGF-induced angiogenesis in vivo[3]. |
| Cell Assay | Evodiamine is dissolved in DMSO and diluted with appropriate medium before use. The evodiamine-inspired new scaffolds are assayed for growth inhibitory activities toward human cancer cell-lines A549 (lung cancer), MDA-MB-435 (breast cancer) and HCT116 (colon cancer) using the MTT assay. Evodiamine and camptithecin are used as reference drugs[1]. |
| Animal Admin | Rats: Twelve healthy male Sprague-Dawley rats are randomly divided into 2 groups: the control group (received oral 10 mg/kg dapoxetine alone) and the combination group (10 mg/kg dapoxetine orally co-administered with 100 mg/kg evodiamine). The plasma concentration of dapoxetine and desmethyl dapoxetine are estimated by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), and different pharmacokinetic parameters are calculated[2]. Mice: A nude mouse xenograft model is established by using 4–6-week-old male BALB/c nude mice. Mice are dosed daily with 20 mg/kg (10 mL/kg) of evodiamine intragastrically, six mice are dosed intraperitoneally with 10 mg/kg of 5-flurouracil (5-FU) twice a week, and six mice are not treated. The tumor volumes are determined by measuring two dimensions, with tumor volume=length×width×width/2. After 2 or 3 weeks of treatment, mice are sacrificed by cervical dislocation under anesthesia with ether, and the tumor tissues are collected[3]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 575.1±50.0 °C at 760 mmHg |
| Melting Point | 263 - 265ºC |
| Molecular Formula | C19H17N3O |
| Molecular Weight | 303.358 |
| Flash Point | 301.6±30.1 °C |
| Exact Mass | 303.137177 |
| PSA | 39.34000 |
| LogP | 1.64 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.764 |
| Storage condition | 2-8°C |
| Hazard Codes | T |
|---|---|
| Risk Phrases | R25 |
| Safety Phrases | S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| Hazard Class | 6.1 |
| HS Code | 2933990090 |
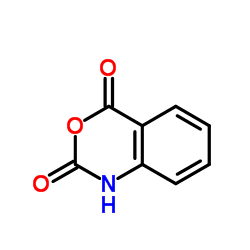
| Precursor 6 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

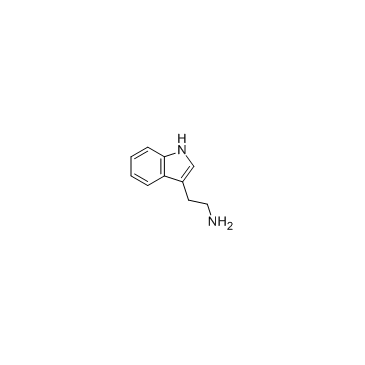
![2-amino-N-[2-(1H-indol-3-yl)ethyl]benzamide structure](https://image.chemsrc.com/caspic/413/33284-02-5.png)
![3-[2-(1H-indol-3-yl)ethyl]quinazolin-4-one structure](https://image.chemsrc.com/caspic/446/60941-86-8.png)