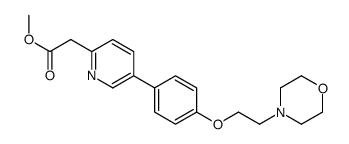
897016-82-9
| Name | N-benzyl-2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]acetamide |
|---|---|
| Synonyms |
KX2-391
N-Benzyl-2-(5-{4-[2-(4-morpholinyl)ethoxy]phenyl}-2-pyridinyl)acetamide N-benzyl-2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)acetamide pound not KX2391 pound not KX2 391 UNII-4V9848RS5G 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-N-benzylacetamide 2-Pyridineacetamide, 5-[4-[2-(4-morpholinyl)ethoxy]phenyl]-N-(phenylmethyl)- N-benzyl-2-{5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl}acetamide cc-266 KX01 |
| Description | KX2-391 is an inhibitor of Src that targets the peptide substrate site of Src, with GI50 of 9-60 nM in cancer cell lines. |
|---|---|
| Related Catalog | |
| Target |
GI50: 9 nM (Src, in HuH7 cells), 13 nM (Src, in PLC/PRF/5 cells), 26 nM (Src, in Hep3B cells), 60 nM (Src, in HepG2 cells)[1] |
| In Vitro | KX2-391 is a Src inhibitor that is directed to the Src substrate pocket. KX2-391 shows steep dose-response curves against Huh7 (GI50=9 nM), PLC/PRF/5 (GI50=13 nM), Hep3B (GI50=26 nM), and HepG2 (GI50=60 nM), four hepatic cell cancer (HCC) cell lines[1]. KX2-391 is found to inhibit certain leukemia cells that are resistant to current commercially available drugs, such as those derived from chronic leukemia cells with the T3151 mutation. KX2-391 is evaluated in engineered Src driven cell growth assays inNIH3T3/c-Src527F and SYF/c-Src527F cells and exhibits GI50 with 23 nM and 39 nM, respectively[2]. |
| In Vivo | Orally administered KX2-391 is shown to inhibit primary tumor growth and to suppress metastasis, in pre-clinical animal models of cancer[2]. |
| Cell Assay | Liver cell lines including Huh7, PLC/PRF/5, Hep3B, and HepG2 are routinely cultured and maintained in basal medium containing 2% fetal bovine serum (FBS) at 37°C and 5% CO2. Cells are seeded at 4.0×103/190 μL and 8.0×103/190 μL per well of 96-well plate in basal medium containing 1.5% FBS. These are cultured overnight at 37°C and 5% CO2 prior to the addition of KX2-391, at concentrations ranging from 6,564 to 0.012 nM in triplicates. Treated cells are incubated for 3 days. Ten μLs of 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL) is then added to each well on day 3 and cells incubated for 4 hours. The formazan product is dissolved with 10% SDS in dilute HCl. Optical density at 570 nm is measured. For comparison of activity and potency, parallel experiments are performed using KX2-391. Growth inhibition curves, 50% inhibition concentration (GI50), and 80% inhibition concentration (GI80) are determined using GraphPad Prism 5 statistical software. Data are normalized to represent percentage of maximum response as well as reported in optical density at wavelength of 570 nm (OD570) signal format[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 680.9±55.0 °C at 760 mmHg |
| Molecular Formula | C26H29N3O3 |
| Molecular Weight | 431.527 |
| Flash Point | 365.6±31.5 °C |
| Exact Mass | 431.220886 |
| PSA | 67.18000 |
| LogP | 1.97 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.588 |
| Storage condition | -20℃ |
| Hazard Codes | N |
|---|
|
~90% 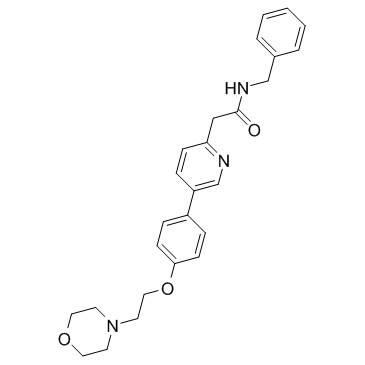
897016-82-9 |
| Literature: KINEX PHARMACEUTICALS, LLC Patent: WO2008/82637 A1, 2008 ; Location in patent: Page/Page column 77-78; 83 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |