531-44-2
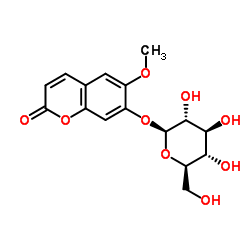
| Name | scopolin |
|---|---|
| Synonyms |
Scopolin
2H-1-Benzopyran-2-one, 7-(β-D-glucopyranosyloxy)-6-methoxy- Scopoletin glucoside 7-(β-D-glucopyranosoyloxy)-6-methoxy-2H-1-benzopyran-2-one 2H-1-Benzopyran-2-one, 7- (β-D-glucopyranosyloxy)-6-methoxy- 5Mg 7-(b-D-Glucopyranosyloxy)-6-methoxy-2H-1-benzopyran-2-one Scopoletin 7-O-Glucoside 6-Methoxy-2-oxo-2H-chromen-7-yl β-D-glucopyranoside 2'-β-D-glucosyl scopolin Murrayin Scopolin (8CI) CPD-678 Scopoletin 7-glucoside 7β-D-Glucopyranosyloxy-6-methoxycoumarin Scopoline Scopoloside |
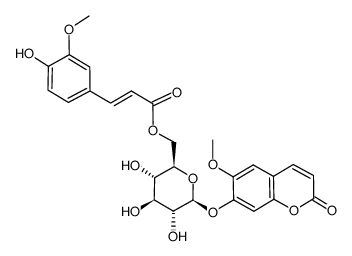
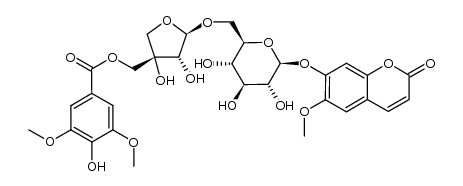
| Description | Scopolin is a coumarin isolated from Arabidopsis thaliana (Arabidopsis) roots[1]. Scopolin attenuated hepatic steatosis through activation of SIRT1-mediated signaling cascades[2]. |
|---|---|
| Related Catalog | |
| Target |
SIRT1 |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 650.2±55.0 °C at 760 mmHg |
| Melting Point | 217ºC |
| Molecular Formula | C16H18O9 |
| Molecular Weight | 354.309 |
| Flash Point | 241.5±25.0 °C |
| Exact Mass | 354.095093 |
| PSA | 138.82000 |
| LogP | -1.87 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.639 |
|
~% 
531-44-2 |
| Literature: Chemistry of Natural Compounds, , vol. 18, # 6 p. 654 - 657 Khimiya Prirodnykh Soedinenii, , vol. 18, # 6 p. 691 - 695 |
|
~% 
531-44-2 |
| Literature: Chemistry of Natural Compounds, , vol. 16, # 2 p. 125 - 128 Khimiya Prirodnykh Soedinenii, , vol. 16, # 2 p. 168 - 172 |
|
~% 
531-44-2 |
| Literature: Angewandte Chemie - International Edition, , vol. 45, # 21 p. 3534 - 3538 |
|
~% 
531-44-2 |
| Literature: Song, Shuang; Li, Yixiu; Feng, Ziming; Jiang, Jianshuang; Zhang, Peicheng Journal of Natural Products, 2010 , vol. 73, # 2 p. 177 - 184 |
| Precursor 4 | |
|---|---|
| DownStream 3 | |