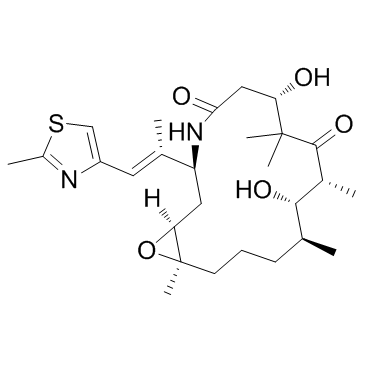
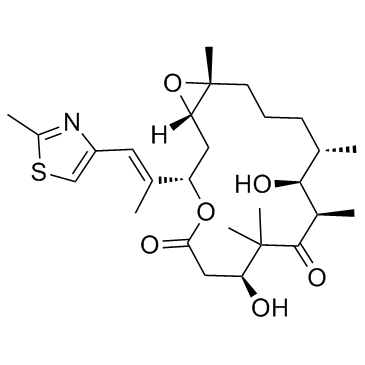
219989-84-1
| Name | ixabepilone |
|---|---|
| Synonyms |
15-aza-EpoB
[14C]-Ixabepilone Ixempra Ixabepilone [INN] 16-aza-epothilone B 17-Oxa-4-azabicyclo[14.1.0]heptadecane-5,9-dione, 7,11-dihydroxy-8,8,10,12,16-pentamethyl-3-[(E)-1-methyl-2-(2-methyl-4-thiazolyl)ethenyl]-, (1S,3S,7S,10R,11S,12S,16R)- [1S-[1R*,3R*(E),7R*,10S*,11R*,12R*,16S*]]-7,11-dihydroxy-8,8,10,12,16-pentamethyl-3-[1-methyl-2-(2-methyl-4-thiazolyl)ethenyl]-4-aza-17-oxabicyclo[14.1.0]heptadecane-5,9-dione (1S,3S,7S,10R,11S,12S,16R)-7,11-dihydroxy-8,8,10,12,16-pentamethyl-3-[(1E)-1-(2-methyl-1,3-thiazol-4-yl)prop-1-en-2-yl]-17-oxa-4-azabicyclo[14.1.0]heptadecane-5,9-dione (1S,3S,7S,10R,11S,12S,16R)-7,11-dihydroxy-8,8,10,12,16-pentamethyl-3-[(E)-1-(2-methyl-1,3-thiazol-4-yl)prop-1-en-2-yl]-17-oxa-4-azabicyclo[14.1.0]heptadecane-5,9-dione Azaepothilone B Ixabepilone (1S,3S,7S,10R,11S,12S,16R)-7,11-dihydroxy-8,8,10,12,16-pentamethyl-3-[(E)-1-methyl-2-(2-methyl-1,3-thiazol-4-yl)ethenyl]-17-oxa-4-azabicyclo[14.1.0]heptadecane-5,9-dione (1S,3S,7S,10R,11S,12S,16R)-7,11-Dihydroxy-8,8,10,12,16-pentamethyl-3-[(1E)-1-(2-methyl-1,3-thiazol-4-yl)-1-propen-2-yl]-17-oxa-4-azabicyclo[14.1.0]heptadecane-5,9-dione (1S,3S,7S,10R,11S,12S,16R)-7,11-dihydroxy-8,8,10,12,16-pentamethyl-3-[(E)-1-methyl-2-(2-methylthiazol-4-yl)vinyl]-17-oxa-4-azabicyclo[14.1.0]hepta-decane-5,9-dione Aza-epothilone B |
| Description | Ixabepilone is an orally bioavailable microtubule inhibitor, which binds to tubulin and promotes tubulin polymerization and microtubule stabilization, thereby arrests cells in the G2-M phase of the cell cycle and induces tumor cell apoptosis. |
|---|---|
| Related Catalog | |
| In Vitro | BMS-247550 is a highly potent cytotoxic agent capable of killing cancer cells at low nanomolar concentrations and retains its antineoplastic activity against human cancers that are naturally insensitive to paclitaxel or that have developed resistance to paclitaxel[1]. |
| In Vivo | BMS-247550 demonstrates antitumor activity that is superior to paclitaxel in both paclitaxel-resistant and -sensitive tumors. BMS-247550 is more efficacious than paclitaxel in all five paclitaxel-resistant tumors evaluated in this study (four human and one murine): the clinically derived paclitaxel resistant Pat-7 ovarian carcinoma, the A2780Tax ovarian carcinoma that is resistant to paclitaxel because of tubulin mutations, the HCT116/VM46 MDR colon carcinoma, the clinically derived paclitaxel-resistant Pat-21 breast carcinoma, and the murine fibrosarcoma M5076. Against three paclitaxel-sensitive human tumor xenografts, BMS-247550 produces antitumor activity equivalent to paclitaxel: A2780 human ovarian carcinoma, HCT116, and LS174T human colon carcinoma[1]. |
| Kinase Assay | The potency with which BMS-247550 and paclitaxel polymerize tubulin isolated from calf brain is evaluated by Published techniques. Briefly, different concentrations of paclitaxel or BMS-247550 in polymerization buffer [0.1 M mes, 1 mM EGTA, 0.5 mM MgCl2 (pH 6.6)] are added to tubulin in polymerization buffer at 37°C in microcuvette wells of a Beckman. Model DU 7400 UV spectrophotometer. A final microtubule protein concentration of 1.0 mg/mL and compound concentrations of generally 2.5, 5.0, and 10 μM are used. Initial slopes of absorbance (A280 nM) change, measured every 10 s, are calculated by the software program accompanying the instrument. |
| Cell Assay | HCT116 cells from cultures are collected by trypsinization after 1, 2, 4, 8, 16, and 24 h exposure to 7.5 nm of BMS-247550. Cells are pelleted and fixed in 80% ethanol at −20°C. After an overnight storage at −20°C, cells are rehydrated with PBS buffer and DNA stain by incubation with propidium iodide (5 μg/mL) in 0.1% RNase for 15-30 min. Fluorescence-activated cell sorter acquisition is performed using the FACS Calibur instrument and analysis is done using Cellquest and Modfit software. |
| References |
[1]. John T. Hunt Discovery of Ixabepilone. Mol Cancer Ther February 2009 8; 275 |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 697.8±55.0 °C at 760 mmHg |
| Molecular Formula | C27H42N2O5S |
| Molecular Weight | 506.698 |
| Flash Point | 375.8±31.5 °C |
| Exact Mass | 506.281433 |
| PSA | 140.29000 |
| LogP | 1.77 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.533 |
| Storage condition | -20 °C |
| Hazard Codes | Xi |
|---|
| Precursor 8 | |
|---|---|
| DownStream 0 | |