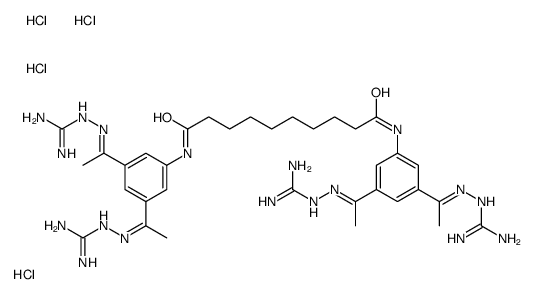
164301-51-3
| Name | N,N'-bis[3,5-bis[(E)-N-(diaminomethylideneamino)-C-methylcarbonimidoyl]phenyl]decanediamide,tetrahydrochloride |
|---|---|
| Synonyms |
Semapimod HCl
Decanediamide,N,N'-bis(3,5-bis(1-((aminoiminomethyl)hydrazono)ethyl)phenyl)-,tetrahydrochloride CNI 1493 AXD 455 N,N'-Bis(3,5-bis(1-((aminoiminomethyl)hydrazono)ethyl)phenyl)decanediamide tetrahydrochloride Semapimod tetrahydrochloride Semapimod hydrochloride |
| Description | Semapimod tetrahydrochloride (CNI-1493), an inhibitor of proinflammatory cytokine production, can inhibit TNF-α, IL-1β, and IL-6. Semapimod tetrahydrochloride inhibits TLR4 signaling (IC50≈0.3 μM). Semapimod tetrahydrochloride inhibits p38 MAPK and nitric oxide production in macrophages. Semapimod tetrahydrochloride has potential in a variety of inflammatory and autoimmune disorders[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Semapimod tetrahydrochloride leads to a significant decrease of p38-MAPK phosphorylation in macrophages, proinflammatory gene expression of macrophage inflammatory protein-1alpha, interleukin-6, monocyte chemoattractant protein-1, and intercellular adhesion molecule-1, and neutrophil infiltration. Semapimod tetrahydrochloride completely abrogated nitric oxide production within the tunica muscularis[2]. Semapimod tetrahydrochloride desensitizes TLR signaling via its effect on the TLR chaperone gp96. Semapimod tetrahydrochloride inhibits ATP-binding and ATPase activities of gp96 in vitro (IC50≈0.2-0.4 μM). Semapimod tetrahydrochloride desensitizes TLR signaling via its effect on the TLR chaperone gp96[3]. |
| In Vivo | Semapimod tetrahydrochloride (5 mg/kg; i.p; daily for 2 weeks) ameliorates endothelial dysfunction in Obese Zucker (OZ) rats[1]. Semapimod tetrahydrochloride restores AM-induced akt phosphorylation and cGMP production in OZ rats[1]. Animal Model: Male OZ rats[1] Dosage: 5 mg/kg Administration: I.p; daily for 2 weeks Result: Restored endothelium-dependent vasorelaxation in OZ rats. |
| References |
| Boiling Point | 1025.8ºC at 760mmHg |
|---|---|
| Molecular Formula | C34H56Cl4N18O2 |
| Molecular Weight | 890.74000 |
| Flash Point | 574.2ºC |
| Exact Mass | 888.35900 |
| PSA | 362.22000 |
| LogP | 11.48360 |
| Vapour Pressure | 0mmHg at 25°C |
| Hazard Codes | Xi |
|---|