702681-67-2
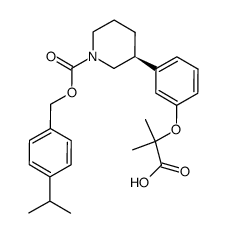
| Name | (S)-3-[3-(1-carboxy-1-methyl-ethoxy)-phenyl]-piperidine-1-carboxylic acid 4-isopropyl-benzyl ester |
|---|
| Description | CP-868388 free base is a potent, selective and orally active PPARα agonist with a Ki value of 10.8 nM. CP-868388 free base has little or no affinity for PPARβ (Ki of 3.47 μM) and PPARγ. CP-868388 free base has hypolipidemic and anti-inflammatory actions[1]. |
|---|---|
| Related Catalog | |
| Target |
hPPARα:10.8 nM (Ki) hPPARγ:3.47 μM (Ki) |
| In Vitro | CP-868388 (0-1 mM) displays robust and dose-dependent recruitment of SRC-1 (EC50 of 4.7 nM) and PGC-1α peptide[1]. CP-868388 demonstrate robust and selective transcriptional activation of PPARα with an EC50 of 18 nM in HepG2 cells[1]. |
| In Vivo | CP-868388 (0-3 mg/kg; oral gavage; once daily; for 2 days; male B6/CBF1J mice) treatment shows a robust and highly significant decrease in circulating plasma triglycerides. Triglyceride lowering is dose-dependent with the greatest efficacy achieved at the 3.0 mg/kg dose, with triglyceride decreases of ~50%[1]. Animal Model: Male B6/CBF1J mice[1] Dosage: 0 mg/kg, 0.3 mg/kg, 1 mg/kg, 3 mg/kg Administration: Oral gavage; once daily; for 2 days Result: Demonstrated a robust and highly significant decrease in circulating plasma triglycerides. |
| References |
| Molecular Formula | C26H33NO5 |
|---|---|
| Molecular Weight | 439.54400 |
| Exact Mass | 439.23600 |
| PSA | 76.07000 |
| LogP | 5.50610 |
| Symbol |


GHS06, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H410 |
| Precautionary Statements | P273-P301 + P310-P501 |
| RIDADR | UN 2811 6.1 / PGIII |