637-32-1
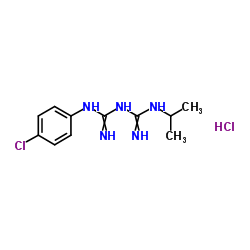
| Name | Proguanil hydrochloride |
|---|---|
| Synonyms |
Imidodicarbonimidic diamide, N-(4-chlorophenyl)-N'-(1-methylethyl)-, monohydrochloride
Biguanide, 1- (p-chlorophenyl)-5-isopropyl-, monohydrochloride N-(4-chlorophenyl)-N'-(1-methylethyl)imidodicarbonimidic diamide hydrochloride diamide N-(4-chlorophényl)-N'-(1-méthyléthyl)imidodicarbonimidique chlorhydrate Paludrin proguanil hydrochloride Paludrine N-(4-Chlorophenyl)-N'-isopropylimidodicarbonimidic diamide hydrochloride (1:1) Drinupal hydrochloride Tirian hydrochloride Palusil hydrochloride Imidodicarbonimidic diamide, N-(4-chlorophenyl)-N'-(1-methylethyl)-, hydrochloride (1:1) N-(4-chlorophenyl)-N'-propan-2-ylimidodicarbonimidic diamide hydrochloride (1:1) N-(4-Chlorphenyl)-N'-(1-methylethyl)imidodicarbonimidic diamidhydrochlorid |
| Description | Proguanil hydrochloride, an antimalarial prodrug, is metabolized to the active metabolite Cycloguanil (HY-12784). Proguanil hydrochloride is a dihydrofolate reductase (DHFR) inhibitor[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Proguanil per se has only weak antimalarial activity in vitro (IC50=2.4-19 μM), and its effectiveness depends on the active metabolite Cycloguanil (IC50=0.5-2.5 nM). The Cycloguanil is a dihydrofolate reductase (DHFR) inhibitor. The combination of Atovaquone and Proguanil is synergistic in vitro. Both drugs also have activity against gametocytes and pre-erythrocytic (hepatic) stages of malaria parasites[1]. Proguanil acts as a biguanide rather than as its metabolite Cycloguanil (a parasite dihydrofolate reductase [DHFR] inhibitor) to enhance the Atovaquone effect. Proguanil-mediated enhancement is specific for Atovaquone, since the effects of other mitochondrial electron transport inhibitors, such as Myxothiazole and Antimycin, are not altered by inclusion of Proguanil[2]. 5-HT3 receptor responses are reversibly inhibited by Proguanil, the metabolite 4-chlorophenyl-1-biguanide (CPB) and the active metabolite Cycloguanil (CG), with an IC50 of 1.81, 1.48 and 4.36 μM, respectively[3]. |
| In Vivo | Proguanil (p.o.; 2.9 mg/kg body weight; daily for 5 days and 6 weeks respectively) shows mild degenerative changes for five days, while shows severe degenerative changes for six weeks in wistar strain albino rats[4]. Serum testosterone level is significantly decreased for proguanil treatment rats[4]. Administration of Malarone (Atovaquone and Proguanil) to experimentally B. gibsoni infected two dogs in chronic stage and three dogs in acute stage results in decrease in parasitemia, and clinical improvements are observed[5]. |
| References |
| Boiling Point | 402.7ºC at 760 mmHg |
|---|---|
| Melting Point | 249-251ºC |
| Molecular Formula | C11H17Cl2N5 |
| Molecular Weight | 290.192 |
| Flash Point | 197.4ºC |
| Exact Mass | 289.086090 |
| PSA | 83.79000 |
| LogP | 4.06530 |
| Storage condition | -20°C Freezer |
| Water Solubility | acetonitrile: water: ~1mg/mL (60/40) |
|
SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifiers Product name: Proguanil Hydrochloride REACH No.: A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline.
CAS-No.: 637-32-1 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Laboratory chemicals, Manufacture of substances SECTION 2: Hazards identification Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Acute toxicity, Oral (Category 3), H301 For the full text of the H-Statements mentioned in this Section, see Section 16. Classification according to EU Directives 67/548/EEC or 1999/45/EC T ToxicR25 For the full text of the R-phrases mentioned in this Section, see Section 16. Label elements Labelling according Regulation (EC) No 1272/2008 Pictogram Signal wordDanger Hazard statement(s) H301Toxic if swallowed. Precautionary statement(s) P301 + P310IF SWALLOWED: Immediately call a POISON CENTER or doctor/ physician. Supplemental Hazardnone Statements Other hazards - none SECTION 3: Composition/information on ingredients Substances Synonyms: Chlorguanide hydrochloride N-(4-Chlorophenyl)-N'-(1-methylethyl)imidodicarbonimidic diamide hydrochloride Formula: C11H16ClN5.HCl Molecular Weight: 290,19 g/mol CAS-No.: 637-32-1 EC-No.: 211-283-7 Hazardous ingredients according to Regulation (EC) No 1272/2008 ComponentClassificationConcentration PROGUANIL HYDROCHLORIDE Acute Tox. 3; H301- Hazardous ingredients according to Directive 1999/45/EC ComponentClassificationConcentration PROGUANIL HYDROCHLORIDE T, R25- For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16 SECTION 4: First aid measures Description of first aid measures General advice Consult a physician. Show this safety data sheet to the doctor in attendance. If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contact Wash off with soap and plenty of water. Take victim immediately to hospital. Consult a physician. In case of eye contact Flush eyes with water as a precaution. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. Most important symptoms and effects, both acute and delayed The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11 Indication of any immediate medical attention and special treatment needed no data available SECTION 5: Firefighting measures Extinguishing media Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special hazards arising from the substance or mixture Carbon oxides, nitrogen oxides (NOx), Hydrogen chloride gas Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information no data available SECTION 6: Accidental release measures Personal precautions, protective equipment and emergency procedures Wear respiratory protection. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8. Environmental precautions Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Methods and materials for containment and cleaning up Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed containers for disposal. Reference to other sections For disposal see section 13. SECTION 7: Handling and storage Precautions for safe handling Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire protection. For precautions see section 2.2. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature: 2 - 8 °C Store with desiccant. Specific end use(s) A part from the uses mentioned in section 1.2 no other specific uses are stipulated SECTION 8: Exposure controls/personal protection Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls Avoid contact with skin, eyes and clothing. Wash hands before breaks and immediately after handling the product. Personal protective equipment Eye/face protection Face shield and safety glasses Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Body Protection Complete suit protecting against chemicals, The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection Where risk assessment shows air-purifying respirators are appropriate use a full-face particle respirator type N99 (US) or type P2 (EN 143) respirator cartridges as a backup to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air respirator. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Control of environmental exposure Prevent further leakage or spillage if safe to do so. Do not let product enter drains. SECTION 9: Physical and chemical properties Information on basic physical and chemical properties a) AppearanceForm: solid b) Odourno data available c) Odour Thresholdno data available d) pHno data available e) Melting point/freezingno data available point f) Initial boiling point and no data available boiling range g) Flash pointno data available h) Evapouration rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- log Pow: 2,270 octanol/water p) Auto-ignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available SECTION 10: Stability and reactivity Reactivity no data available Chemical stability Stable under recommended storage conditions. Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials no data available Hazardous decomposition products Other decomposition products - no data available In the event of fire: see section 5 SECTION 11: Toxicological information Information on toxicological effects Acute toxicity LD50 Oral - rat - 58 mg/kg LD50 Oral - mouse - 27 mg/kg LD50 Oral - rabbit - 150 mg/kg LD50 Intravenous - rat - 33 mg/kg Remarks: Behavioral:Altered sleep time (including change in righting reflex). Behavioral:Convulsions or effect on seizure threshold. LD50 Intravenous - mouse - 23 mg/kg Remarks: Behavioral:Altered sleep time (including change in righting reflex). Behavioral:Convulsions or effect on seizure threshold. LD50 Subcutaneous - mouse - 35 mg/kg LD50 Intravenous - rabbit - 44,85 mg/kg Remarks: Behavioral:Altered sleep time (including change in righting reflex). LD50 Intraperitoneal - guinea pig - 15 mg/kg LD50 Intravenous - guinea pig - 39,51 mg/kg Remarks: Behavioral:Altered sleep time (including change in righting reflex). Behavioral:Convulsions or effect on seizure threshold. Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitisation no data available Germ cell mutagenicity Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Additional Information RTECS: DU1750000 To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. SECTION 12: Ecological information Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment PBT/vPvB assessment not available as chemical safety assessment not required/not conducted Other adverse effects no data available SECTION 13: Disposal considerations Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. Contaminated packaging Dispose of as unused product. SECTION 14: Transport information UN number ADR/RID: 2811IMDG: 2811IATA: 2811 UN proper shipping name ADR/RID: TOXIC SOLID, ORGANIC, N.O.S. IMDG: TOXIC SOLID, ORGANIC, N.O.S. (PROGUANIL HYDROCHLORIDE) IATA:Toxic solid, organic, n.o.s. (PROGUANIL HYDROCHLORIDE) Transport hazard class(es) ADR/RID: 6.1IMDG: 6.1IATA: 6.1 Packaging group ADR/RID: IIIIMDG: IIIIATA: III Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available SECTION 15: Regulatory information This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006. Safety, health and environmental regulations/legislation specific for the substance or mixture no data available Chemical Safety Assessment For this product a chemical safety assessment was not carried out SECTION 16: Other information Full text of H-Statements referred to under sections 2 and 3. Acute Tox.Acute toxicity H301Toxic if swallowed. Full text of R-phrases referred to under sections 2 and 3 T Toxic R25 Toxic if swallowed. Further information Copyright 2013 Co. LLC. License granted to make unlimited paper copies for internal use only. The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Corporation and its Affiliates shall not be held liable for any damage resulting from handling or from contact with the above product. See and/or the reverse side of invoice or packing slip for additional terms and conditions of sale. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| Hazard Codes | T |
| Risk Phrases | 25 |
| Safety Phrases | 45 |
| RIDADR | UN 3249 |
| RTECS | DU1750000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |