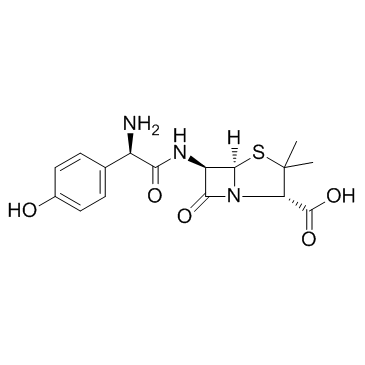
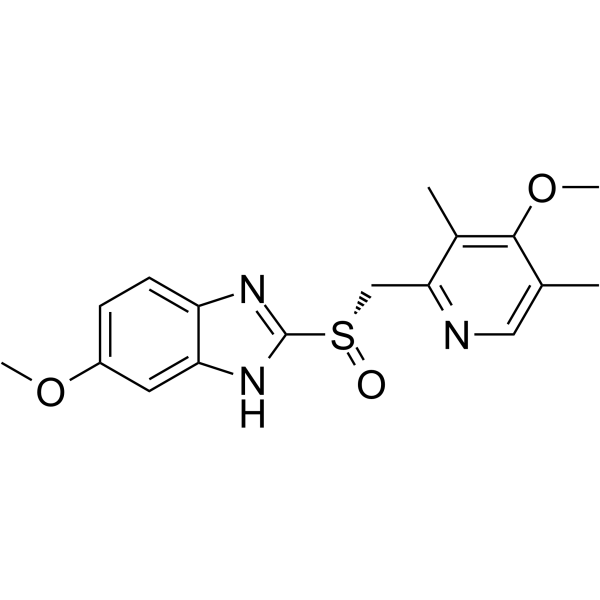
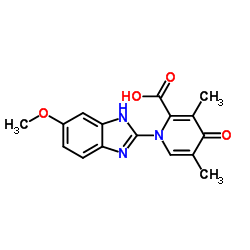
73590-58-6
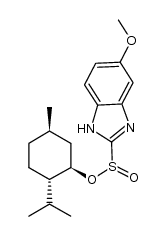
| Name | 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-benzimidazole |
|---|---|
| Synonyms |
5-methoxy-2-[(4-methoxy-3,5-dimethyl-pyridin-2-yl)-methylsulfinyl]benzimidazole
5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole Omepral (RS)-5-Methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridylmethylsulphinyl)benzimidazole elgam ANTRA MEPRAL Zoltum 1H-Benzimidazole, 5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl)- [14C]-Omeprazole PRILOSEC (-)-5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole zimor Losec Omeprazen Pepticum prysma 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-benzimidazole EINECS 201-212-8 (±)-Omeprazole MFCD00083192 Omeprazole 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulphinyl]-1H-benzimidazole aulcer 6-Methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-benzimidazole 5-Methoxy-2-{[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl}-1H-benzimidazole 1H-Benzimidazole, 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]- sulfonium, hydroxy(6-methoxy-1H-benzimidazol-2-yl)[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]-, inner salt ulcsep 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methylsulphinyl]-1H-benzimidazole Miol Gastrogard |
| Description | Omeprazole(Prilosec) is a proton pump inhibitor used in the treatment of dyspepsia.Target: Proton PumpOmeprazole is a proton pump inhibitor used in the treatment of dyspepsia, peptic ulcer disease, gastroesophageal reflux disease, laryngopharyngeal reflux, and Zollinger-Ellison syndrome. Omeprazole virtually eliminated intragastric acidity in all patients: the median 24 hour intragastric pH rose from 1.4 to 5.3 and the mean hourly hydrogen ion activity fell from 38.50 to 1.95 mmol(mEq)/1 (p less than 0.001). This inhibition of 24 hour intragastric acidity is more profound than that previously reported with either cimetidine 1 g daily or ranitidine 300 mg daily [1]. The pharmacokinetics of omeprazole were studied in a group of healthy male subjects after single and repeated oral doses of 30 and 60 mg. Absorption of omeprazole from its enteric-coated formulation was unpredictable. There was a highly significant increase in the area under the plasma concentration time curve (AUC) after repeated dosing. Omeprazole increases its own relative availability following repeated dosing. This may be due to inhibition of gastric acid secretion by omeprazole which is an acid-labile compound [2].Clinical indications: Duodenal ulcer; Endocrine tumor; Esophagitis; Gastroesophageal reflux; Helicobacter pylori infection; Stomach ulcer; Zollinger-Ellison syndromeToxicity: Symptoms of overdose include confusion, drowsiness, blurred vision, tachycardia, nausea, diaphoresis, flushing, headache, and dry mouth. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 600.0±60.0 °C at 760 mmHg |
| Melting Point | 156ºC |
| Molecular Formula | C17H19N3O3S |
| Molecular Weight | 345.416 |
| Flash Point | 316.7±32.9 °C |
| Exact Mass | 345.114716 |
| PSA | 96.31000 |
| LogP | 2.17 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.669 |
| Storage condition | 2-8°C |
| Stability | Stable, but hygroscopic and photosensitive. Incompatible with strong oxidizing agents. Store in the dark. |
| Water Solubility | H2O: 0.5 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | DD9087000 |
| Precursor 8 | |
|---|---|
| DownStream 7 | |




![5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]thio]-1H-benzimidazole N-Oxide structure](https://image.chemsrc.com/caspic/092/142885-92-5.png)