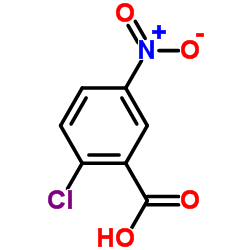
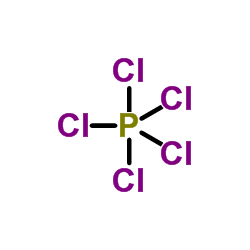
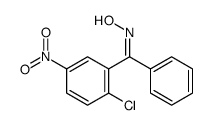
22978-25-2
| Name | 2-Chloro-5-nitro-N-4-phenylbenzamide |
|---|---|
| Synonyms |
MFCD01215270
Benzamide, 2-chloro-5-nitro-N-phenyl- 2-Chloro-5-nitro-N-phenylbenzamide GW-9662 GW9662 |
| Description | GW9662 is a potent and selective PPARγ antagonist with an IC50 of 3.3 nM, showing 10 and 1000-fold selectivity over PPARα and PPARδ, respectively. |
|---|---|
| Related Catalog | |
| Target |
PPARγ:3.3 nM (IC50) PPARα:32 nM (IC50) PPARδ:2000 nM (IC50) |
| In Vitro | GW9662 inhibits radioligand binding to PPARγ, PPARα, and PPARδ with pIC50s of 8.48±0.27 (IC50=3.3 nM; n=10), 7.49±0.17 (IC50=32 nM; n=9), and 5.69±0.17 (IC50=2000 nM; n=3), respectively. GW9662 has nanomolar IC50 versus PPARγ and is 10- and 600-fold less potent in binding experiments using PPARα and PPARδ, respectively. In cell-based reporter assays, GW9662 is a potent and selective antagonist of full-length PPARγ[1]. Co-treatment with both 50 μM Rosiglitazone and 10 μM GW9662 results in statistically lower viable cell numbers after 7 days when compared to treatment with either 50 μM rosiglitazone (P=0.001) or 10 μM GW9662 (P=0.01) alone[2]. |
| In Vivo | Bone marrow (BM) nucleated cell counts in both BADGE- and GW9662(1 mg/kg, i.p.)-treated mice are significantly higher than counts in the aplastic anemia (AA) group[3]. GW9662 (1 mg/kg, i.p.) largely attenuates the renoprotective effects of Lipopolysaccharide (LPS) in the rat[4]. |
| Cell Assay | Cells (MCF7, MDA-MB-231, MDA-MB-468) are plated in 96-well plates at a density of 1×103 cells per well in RPMI medium. After overnight incubation to allow for cell attachment, the medium is removed and replaced with fresh medium containing varying concentrations of Rosiglitazone (1-100 μM), GW9662 (100 nM-50 μM) or solvent (DMSO) alone. MDA-MB-231 cells are also subjected to combinations of Rosiglitazone (10, 50 μM) and GW9662 (1, 10 μM) added simultaneously. The final concentration of DMSO in all cases does not exceed 0.1% and is not found to be cytotoxic in any of the cell lines tested at this concentration. Chemosensitivity is assessed following a continuous 72 h exposure using a standard MTT assay. |
| Animal Admin | Mice[3] Inbred C57BL/6 (B6, H2b/b), DBA/1J (DBA/1, H2q/q), FVB/NJ (FVB, H2q/q) mice and congenic C.B10-H2b/b/LilMcd (CB10, H2b/b) mice are used. BADGE or GW9662, are administrated by daily intraperitoneal injection at 30 mg/kg for BADGE, or at 1 mg/kg for GW9662, from one day prior to the experiment and continued for up to 2 weeks. In the FVB AA model, some mice are injected with cyclosporine A (CsA, 50 mg/kg/day) starting 1 hour after the LN injection, and continued for 5 days as immunosuppression. At the end of the experiments, the mice are euthanized by CO2 inhalation. Rats[4] Sixty-two male Wistar rats weighing 215 to 315 g are used in this study. Animals are randomly allocated into 6 groups as follows: (1) I/R group: control, rats are administered 10% (v/v) DMSO (vehicle for GW9662, 1 mL /kg, IP) 24 and 12 hours prior to renal I/R, and saline (vehicle for LPS, 1 mL /kg, IP) 24 hours prior to renal I/R (N=12); (2) I/R LPS group: rats are administered 10% (v/v) DMSO (vehicle for GW9662, 1 mL /kg, IP) 24 and 12 hours prior to renal I/R, and LPS (1 mg/kg, IP) 24 hours prior to renal I/R (N=11); (3) I/R GW9662 group: rats are administered GW9662 (1 mg/kg, IP) 24 and 12 hours prior to renal I/R, and saline (vehicle for LPS, 1 mL /kg, IP) 24 hours prior to renal I/R (N=9); (4) I/R LPS+GW9662 group: rats are administered GW9662 (1 mg/kg, IP) 24 and 12 hours prior to renal I/R, and LPS (1 mg/kg, IP) 24 hours prior to renal I/R (N=11); (5) Sham group: rats are subjected to the same surgical procedures as above, except for renal I/R. Rats are administered 10% (v/v) DMSO (vehicle for GW9662, 1 mL /kg, IP) and saline (vehicle for LPS, 1 mL /kg, IP) at times equivalent to those described above (N=12); (6) Sham GW9662 group: rats are subjected to the same surgical procedures as above, except for renal I/R. Rats are administered GW9662 (1 mg/kg, IP) and saline (vehicle for LPS, 1 mL /kg, IP) at times equivalent to those described above (N=7). |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 360.9±32.0 °C at 760 mmHg |
| Melting Point | 171-175 °C(lit.) |
| Molecular Formula | C13H9ClN2O3 |
| Molecular Weight | 276.675 |
| Flash Point | 172.0±25.1 °C |
| Exact Mass | 276.030182 |
| PSA | 74.92000 |
| LogP | 2.76 |
| Appearance | solid | white |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.676 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: 26 mg/mL, soluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H319 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | R36;R43 |
| Safety Phrases | S26-S36/37-S24/25-S22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924299090 |
|
~88% 
22978-25-2 |
| Literature: Henke, Adam; Srogl, Jiri Journal of Organic Chemistry, 2008 , vol. 73, # 19 p. 7783 - 7784 |
|
~66% 
22978-25-2 |
| Literature: Kumar, Anil; Narasimhan, Balasubramanian; Kumar, Devinder Bioorganic and Medicinal Chemistry, 2007 , vol. 15, # 12 p. 4113 - 4124 |
|
~% 
22978-25-2 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 446, p. 225 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |