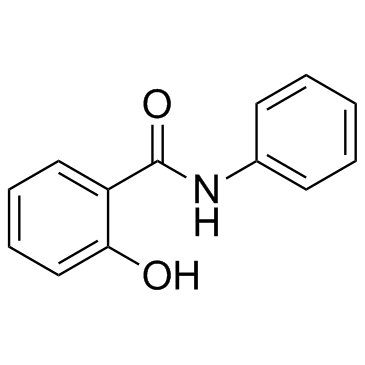
87-17-2
| Name | salicylanilide |
|---|---|
| Synonyms |
2-Phenylaminocarbonylphenol
Salinide Benzamide, 2-hydroxy-N-phenyl- Salicylanilid 2-Hydroxy-N-phenylbenzamide Salifebrin Ansadol salicylamide salicylic acid anilide N-Phenylsalicylamide Hyanilid EINECS 201-727-8 2-hydroxy-N-phenyl-benzamide Aseptolan Salicylanilide MFCD00002212 |
| Description | Salicylanilide demonstrates a wide range of biological activities including antiviral potency which can inhibit HIV virus by targeting HIV-1 integrase or reverse transcriptase. |
|---|---|
| Related Catalog | |
| Target |
HIV-1 integrase, reverse transcriptase[1] |
| In Vitro | Some Salicylanilides and salicylamides could inhibit HIV virus by targeting of HIV-1 integrase or reverse transcriptase. Hepatitis C virus is another virus, which can be potentially afflicted by Salicylanilides on the level of two enzymes-NS3 protease and NS5B RNA polymerase[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 294.3±23.0 °C at 760 mmHg |
| Melting Point | 136-138 °C(lit.) |
| Molecular Formula | C13H11NO2 |
| Molecular Weight | 213.232 |
| Flash Point | 131.8±22.6 °C |
| Exact Mass | 213.078979 |
| PSA | 49.33000 |
| LogP | 3.27 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.676 |
| Water Solubility | SLIGHTLY SOLUBLE |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335-H400 |
| Precautionary Statements | P261-P273-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 2 |
| RTECS | VN7850000 |
| HS Code | 29242995 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |