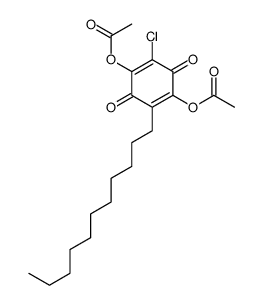
550-24-3
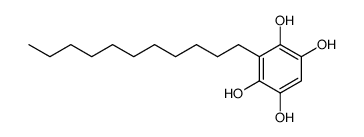
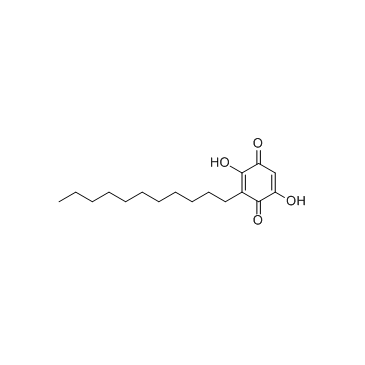
| Name | Embelin |
|---|---|
| Synonyms |
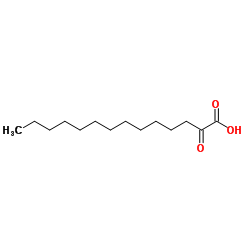
2,5-Dihydroxy-3-undecyl-1,4-benzoquinone
Embelin 2,5-dihydroxy-3-undecylcyclohexa-2,5-diene-1,4-dione 2,5-Cyclohexadiene-1,4-dione, 2,5-dihydroxy-3-undecyl- EINECS 208-979-8 |
| Description | Embelin is a cell-permeable benzoquinone compound that exhibits antitumor properties. Specifically antagonizes XIAP-mediated inhibition of caspase-9 activation by directly targeting the Smac and caspase-9 binding domain BIR3 (IC50 = 4.1 uM in a competitive binding assay with Smac peptide).IC50 value: 4.1 uM [1]Target: XIAPin vitro: Embelin induced activation of caspase-9 and embelin-induced apoptosis was prevented by caspase inhibitors [2]. Treatment with subtoxic doses of Embelin broadly sensitized malignant glioma cells to TRAIL-mediated apoptosis. Notably, human astrocytes were not significantly affected by the combined treatment consisting of Embelin and TRAIL. Combined treatment with Embelin and TRAIL augmented the activation of initiator caspases-8/-9 and effector caspases-3/-7, respectively [3]. in vivo: Embelin inhibited topical edema in the mouse ear, leading to substantial reductions in skin thickness and tissue weight, inflammatory cytokine production, neutrophil-mediated myeloperoxidase activity, and various histopathological indicators. Furthermore, embelin was effective at reducing inflammatory damage induced by chronic TPA exposure [4]. Embelin (10, 30 or 50mg/kg body weight) was administrated daily per oral route for 7days. Embelin significantly attenuated DSS-induced DAI scores and tissue MPO accumulation, which implied that it suppressed weight loss, diarrhea, gross bleeding, and the infiltrations of immune cells. Embelin administration also effectively and dose-dependently prevented shortening of colon length and enlargement of spleen size [5]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.131 |
|---|---|
| Boiling Point | 431.9±45.0 °C at 760 mmHg |
| Melting Point | 145-146 ºC |
| Molecular Formula | C17H26O4 |
| Molecular Weight | 294.386 |
| Flash Point | 229.1±25.2 °C |
| Exact Mass | 294.183105 |
| PSA | 74.60000 |
| LogP | 5.70 |
| Appearance | solid |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.538 |
| Storage condition | Store at +4°C |
| Water Solubility | DMSO: >10 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DK4230000 |
| HS Code | 2914690090 |
|
~% 
550-24-3 |
| Literature: Journal of the American Chemical Society, , vol. 70, p. 74 |
|
~% 
550-24-3 |
| Literature: Journal of the American Chemical Society, , vol. 70, p. 74 |
|
~% 
550-24-3 |
| Literature: Yakugaku Zasshi, , vol. 60, p. 105,113; dtsch. Ref. S. 34, 37 Chem.Abstr., , p. 5070 Yakugaku Zasshi, , vol. 60, p. 650,658 Yakugaku Zasshi, , vol. 61, p. engl. Ref. S. 1, 5 Chem.Abstr., , p. 83 |
|
~% 
550-24-3 |
| Literature: Archiv der Pharmazie (Weinheim, Germany), , vol. 238, p. 22 |
|
~% 
550-24-3 |
| Literature: Archiv der Pharmazie (Weinheim, Germany), , vol. 238, p. 22 |
| Precursor 5 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914690090 |
|---|---|
| Summary | 2914690090 other quinones。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |