16416-33-4
| Name | 1,1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,15,15,16,16,17,17,18,18,19,19,20,20,21,21,22,22,23,23,24,24,25,25,26,26,27,27,28,28,28-octapentacontadeuteriooctacosane |
|---|---|
| Synonyms |
Octacosane-d
Octacosane-d58 MFCD00190493 (H)Octacosane |
| Description | Octacosane-d58 is the deuterium labeled Octacosane[1]. Octacosane is an endogenous metabolite with antibacterial activity. Octacosane shows high cytotoxicity against murine melanoma B16F10-Nex2 cells besides inducing protection against a grafted subcutaneous melanoma. Octacosane has the larvicidal activity against mosquito Culex quinquefasciatus with the LC50 concentration of 7.2 mg/l[2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Density | 0.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 431.7±8.0 °C at 760 mmHg |
| Melting Point | 61-63ºC(lit.) |
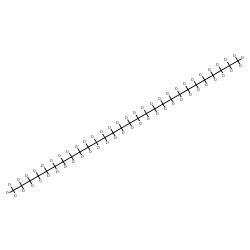
| Molecular Formula | C28D58 |
| Molecular Weight | 453.117 |
| Flash Point | 280.1±8.0 °C |
| Exact Mass | 452.817902 |
| LogP | 15.63 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.450 |
| RIDADR | NONH for all modes of transport |
|---|
| Precursor 1 | |
|---|---|
| DownStream 0 | |