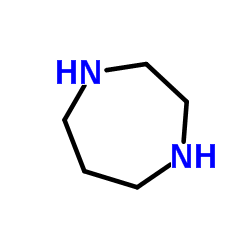
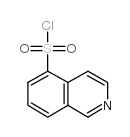
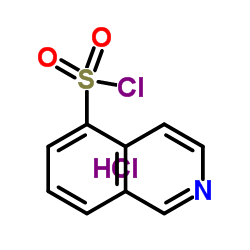
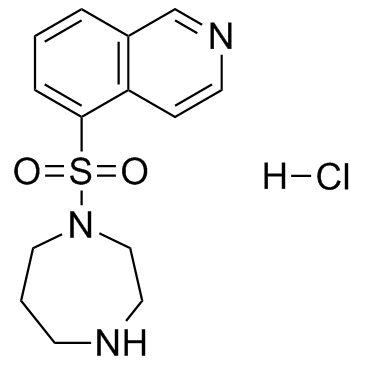
103745-39-7
| Name | fasudil |
|---|---|
| Synonyms |
Fasudilum [inn-latin]
H-1077 dihydrochloride 1-(5-Isoquinolinesulfonyl)-1H-hexahydro-1,4-diazepine,Dihydrochloride 1-(5-ISOQUINOLINE-SULFONYL)-HOMOPIPERAZINE HA 1077,DIHYDROCHLORIDE 5-(1,4-Diazepan-1-ylsulfonyl)isoquinoline HA-1077 DIHYDROCHLORIDE NOVEL VASODILATO R AGE at877 5-(1,4-DIAZEPAN-1-SULFONYL)ISOQUINOLINE Fasudilum 1-(5-Isoquinolinylsulfonyl)homopiperazine Fasudil MFCD00866182 hexahydro-1-(5-isoquinolinylsulfonyl)-1H-1,4-diazepine 1-(5-isoquinolinesulphonyl)-homopiperazine 1-(5-Isoquinolinesulphonyl)homopiperazine N-(5-Isoquinolinesulfonyl)-1,4-perhydrodiazepine (5-ISOQUINOLINESULFONYL)HOMOPIPERAZINE |
| Description | Fasudil is a potent inhibitor of ROCK1, PKA, PKC, and MLCK with Ki of 0.33 μM, 1.0 μM, 9.3 μM and 55 μM, respectively. |
|---|---|
| Related Catalog | |
| Target |
p160ROCK:0.33 μM (Ki) PKA:1 μM (Ki) PKC:9.3 μM (Ki) MLCK:55 μM (Ki) |
| In Vitro | Fasudil has vasodilatory action and occupies the adenine pocket of the ATP-binding site of the enzyme[1]. Fasudil produces a competitive inhibition of the Ca2+-induced contraction of the depolarized rabbit aorta. Fasudil inhibits contractile responses to KCl, phenylephnne (PHE) and prostaglandin (PG) F2a[2]. Fasudil also exhibits vasodilator actions by inhibition of 5-hydroxytryptamine, noradrenaline, histamine, angiotensin, and dopamine induced spiral strips contraction[3]. In addition, Fasudil induces disorganization of actin stress fiber and cell migration inhibition[4]. Fasudil inhibits hepatic stellate cells spreading, the formation of stress fibers, and expression of α-SMA with concomitant suppression of cell growth, but does not induce apoptosis. Fasudil also blocks the LPA-induced phosphorylation of ERK1/2, JNK and p38 MAPK[5]. |
| In Vivo | Fasudil (30 μg) increases CBF by 50% via intra-coronary injection to dogs. Fasudil (0.01, 0.03, 0.1 and 0.3 mg/kg, bolus, i.v.) decreases MBP and increases HR, VBF, CBF, RBF, and FBF. Fasudil (1.0 ng/mL) increases cardiac output. Fasudil via i.v. produces a significant fall in MBP, left ventricular systolic pressure and total peripheral resistance with an increase in HR and cardiac output, but without obvious effect on right atrial pressure, dP/dt or left ventricular minute work in dogs[3]. Fasudil exhibits protectable effects on cardiovascular disease and reduces the activation of JNK and attenuates mitochondrial-nuclear translocation of AIF under ischemic injury[6]. Fasudil (100 mg/kg/day, p.o.) significantly reduces incidence and mean maximum clinical score of EAE in SJL/J mice immunized with PLP p139-151. Fasudil inhibits the proliferative response of splenocytes to the antigen in mice. Fasudil decreases inflammation, demyelination, axonal loss and APP positivein spinal cord of Fasudil-treated mice via p.o. administration[7]. |
| Kinase Assay | Cyclic AMP-dependent protein kinase activity is assayed in a reaction mixture containing, in a final volume of 0.2 mL, 50 mM Tris-HCl (pH 7.0), 10 mM magnesium acetate, 2 mM EGTA, 1 μM cyclic AMP or absence of cyclic AMP, 3.3 to 20 μM [r-32P] ATP (4×105 c.p.m.), 0.5 μg of the enzyme, 100 μg of histone H2B and compound. The mixture is incubated at 30°C for 5 min. The reaction is terminated by adding 1mL of ice-cold 20% trichloroacetic acid after adding 500 μg of bovine serum albumin as a carrier protein. The sample is centrifuged at 3000 r.p.m. for 15min, the pellet is resuspended in ice-cold 10% trichloro-acetic acid solution and the centrifugation-resuspension cycle is repeated three times. The final pellet is dissolved in 1 mL of 1 N NaOH and radioactivity is measured with a liquid scintillation counter[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 506.2±60.0 °C at 760 mmHg |
| Molecular Formula | C14H17N3O2S |
| Molecular Weight | 291.37 |
| Flash Point | 259.9±32.9 °C |
| PSA | 70.68000 |
| LogP | 1.19 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.622 |
| Storage condition | Store at RT |
| Water Solubility | H2O: >200 mg/mL |
| WGK Germany | 3 |
|---|---|
| RTECS | HM4031166 |
| HS Code | 2933990090 |
|
~88% 
103745-39-7 |
| Literature: Asahi Kasei Kogyo Kabushiki Kaisha Patent: US5942505 A1, 1999 ; |
|
~89% 
103745-39-7 |
| Literature: Asahi Kasei Kogyo Kabushiki Kaisha Patent: US4678783 A1, 1987 ; US 4678783 A |
| Precursor 3 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |