54187-04-1
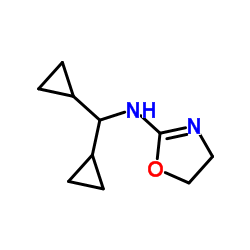
| Name | N-(dicyclopropylmethyl)-4,5-dihydro-1,3-oxazol-2-amine |
|---|---|
| Synonyms |
2-Oxazolamine, N-(dicyclopropylmethyl)-4,5-dihydro-
Rilmenidine S 3341-3 S 3341 N-(Dicyclopropylmethyl)-4,5-dihydro-2-oxazolamine Oxaminozoline hyperium N-(Dicyclopropylmethyl)-4,5-dihydro-1,3-oxazol-2-amine 2-[N-(Dicyclopropylmethyl)amino]oxazoline EINECS 259-021-0 Tenaxum MFCD00865924 |
| Description | Rilmenidine, an innovative antihypertensive agent, is an orally active, selective I1 imidazoline receptor agonist. Rilmenidine is an alpha 2-adrenoceptor agonist. Rilmenidine induces autophagy. Rilmenidine modulates proliferation and stimulates the proapoptotic protein Bax thus inducing the perturbation of the mitochondrial pathway and apoptosis in human leukemic K562 cells[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Rilmenidine (25-100 μM; 24 hours) inhibits K562 cell proliferation[2]. Cell Viability Assay[2] Cell Line: K562 cells Concentration: 25, 50, 100 μM Incubation Time: 24 hours Result: Dose-dependently inhibited K562 colony formation. |
| In Vivo | Rilmenidine-treated N171-82Q mice (i.p.; 4-times a week) displays significant improved forelimb grip strength and all limbs grip strength from 12 to 22 weeks of age[3]. |
| References |
[1]. Reid JL. Rilmenidine: a clinical overview. Am J Hypertens. 2000;13(6 Pt 2):106S-111S. |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 355.5±9.0 °C at 760 mmHg |
| Melting Point | 106 - 107ºC |
| Molecular Formula | C10H16N2O |
| Molecular Weight | 180.247 |
| Flash Point | 168.8±18.7 °C |
| Exact Mass | 180.126266 |
| PSA | 33.62000 |
| LogP | 0.57 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.711 |
| Storage condition | Store at -20°C |
| Water Solubility | H2O: 7.3 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Safety Phrases | S22-S24/25 |
|---|---|
| WGK Germany | 3 |
| RTECS | RP7207400 |
| HS Code | 2934999090 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |