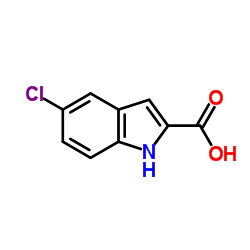
459168-41-3
| Name | 1-[(5-Chloro-1H-indol-2-yl)carbonyl]-4-methyl-piperazine |
|---|---|
| Synonyms |
Methanone, (5-chloro-1H-indol-2-yl)(4-methyl-1-piperazinyl)-
(5-Chloro-1H-indol-2-yl)(4-methyl-1-piperazinyl)methanone JNJ-7777120 (5-chloro-1H-indol-2-yl)(4-methylpiperazin-1-yl)methanone (5-chloro-1H-indol-2-yl)-(4-methylpiperazin-1-yl)methanone 1-((5-chloro-1H-indol-2-yl)carbonyl)-4-methylpiperazine |
| Description | JNJ-7777120 is a selective H4R antagonist with Ki of 4 ±1 nM, exhibits >1000-fold selectivity over the other histamin receptors.IC50 value: 4 ±1 nM (Ki) [1] Target: histamine H4 receptorin vitro: JNJ-7777120 prevents fibronectin-induced lung fibroblast migration, thus suggesting that H4R could represent an attractive target for the development of new drugs for lung fibrosis treatment .[2]in vivo: JNJ 7777120 blocks histamine-induced chemotaxis and calcium influx in mouse bone marrow-derived mast cells. In addition, it can block the histamine-induced migration of tracheal mast cells from the connective tissue toward the epithelium in mice. JNJ 7777120 significantly blocks neutrophil infiltration in a mouse zymosan-induced peritonitis model. [3] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 477.0±45.0 °C at 760 mmHg |
| Molecular Formula | C14H16ClN3O |
| Molecular Weight | 277.749 |
| Flash Point | 242.3±28.7 °C |
| Exact Mass | 277.098175 |
| PSA | 39.34000 |
| LogP | 0.69 |
| Appearance | solid | white |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.656 |
| Storage condition | Store at +4°C |
| Water Solubility | H2O: insoluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~81% 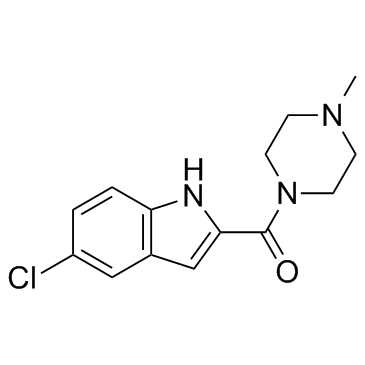
459168-41-3 |
| Literature: Lazewska, Dorota; Karolak-Wojciechowska, Janina; Kolesinska, Beata; Kaminski, Zbigniew; Kiec-Kononowicz, Katarzyna Heterocyclic Communications, 2011 , vol. 17, # 5-6 p. 207 - 210 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |