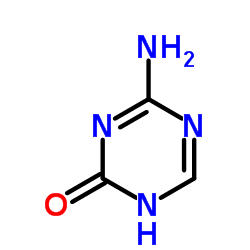
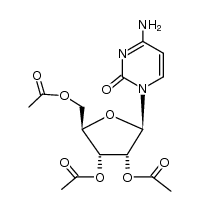
320-67-2
| Name | 5-azacytidine |
|---|---|
| Synonyms |
Vidaza
4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxyméthyl)tétrahydrofuran-2-yl]-1,3,5-triazin-2(1H)-one EINECS 206-280-2 [14C]-Azacitidine 5 AZC 4-Amino-1-b-D-ribofuranosyl-1,3,5-triazine-2(1H)-one 5-AZCR 1,3,5-Triazin-2(1H)-one, 4-amino-1-β-D-ribofuranosyl- 5'-azacytidine azacytidine Ladakamycin Azacitidine AzGR 5-Azacytidine 4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-1,3,5-triazin-2(1H)-one 5-AC 4-amino-1-(b-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one 5-AC-15N4 4-Amino-1-(β-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one 4-Amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydro-2-furanyl]-1,3,5-triazin-2(1H)-one L-β-Ribofuranosyl-5-azacytosine 4-Amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-1,3,5-triazin-2(1H)-on 1,3,5-triazin-2(1H)-one, 4-amino-1-b-D-ribofuranosyl- mylosar Azacytidine, 5- MFCD00006539 5-AZAC |
| Description | 5-Azacytidine is a nucleoside analogue of cytidine that specifically inhibits DNA methylation by trapping DNA methyltransferases. |
|---|---|
| Related Catalog | |
| Target |
DNMT1 Nucleoside Antimetabolite/Analog Autophagy |
| In Vitro | Unmethylated CpG islands associated with a variety of genes become partially or fully methylated in tumors and can be reactivated by 5-Azacytidine[1]. 5-Azacytidine acts as weak inducers of erythroid differentiation of Friend erythroleukemia cells in the same concentration range where they affect DNA methyltransferase activity[2]. 5-Azacytidine inhibits L1210 cells with ID50 and ID90 values of 0.019 and circa 0.15 μg/mL, respectively[3]. |
| In Vivo | TdR-3H incorporation is significantly inhibited when the animals are exposed to 5-Azacitidine (100 mg/kg, i.p.) for 2 hr or longer[3]. |
| Kinase Assay | A crude cell-free extract is isolated from LI 210 cells in culture by suspension of the cells in a given volume of 0.05mol/LTris-HCl buffer, pH 7.4, and sonic extraction with a Biosonik at 70% maximal output for 30 sec. The supernatant is collected after centrifugation at 105,000 × g for 60 min (4°C) in a Model L Spinco ultracentrifuge. The final protein concentration of the cell-free extracts is approximately 3 mg/mL. The extracts are used as the source of enzymes. Ribonucleotide reductase activity is measured. A unit of enzyme is defined as the amount that catalyzed dCMP synthesis at a rate of 1 mμmole/hr. The assay systems for the measurement of pyrimidine nucleoside (CR) and deoxynucleoside (TdR, CdR) kinases are essentially those described by Chu and Fischer. However, reactions are terminated by heating for 2 min in a boiling water bath, and the phosphorylated derivatives are isolated according to the method of Bach. Fifty-jul aliquots are applied to 1-inch discs of diethylaminoethyl paper, which are then placed in counting vials and eluted with 0.5 mL of 0.5 mol/LPCA. After 1 hr, 12 mL of Diotol are added, and the radioactivity is determined. |
| Cell Assay | Twenty mL of cells (circa 1×104 cells/mL) are pipetted into sterilized culture tubes with screw caps and incubated at 37°C overnight. The experiment is initiated by the addition of 1 mL of 5-Azacytidine (5-azaCR) or medium for a given period (from 0 to 240 min) prior to the addition of 1 mL of metabolite (or medium). Cell growth is determined twice a day for 3 days by means of a Model A Coulter counter. To determine IDSO and ID90 values, 5 mL of L1210 cells (5×103 cells/mL) are incubated with the drug at 37°C for 3 days, and cell growth is determined. |
| Animal Admin | For the in vivo experiments, leukemic mice (bearing circa 1×103 cells/animal) are given injections i.p. with 0.2 mL of 5-Azacytidine (5-azaCR) of a given concentration. Two hr later, the reaction is started by injecting 0.5 mL of labeled metabolite (TdR-3H or UR-3H, 10 /μCi/12.5 μg). After 1 hr, animals (3 mice/group) are killed by cervical fracture, and the ascites are treated with heparin, collected, pooled, and then centrifuged immediately in a Sorvall refrigerated centrifuge Model R2C-B at 800×g for 10 min (4°C). |
| References |
| Density | 2.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 534.5±60.0 °C at 760 mmHg |
| Melting Point | 226-232 °C (dec.)(lit.) |
| Molecular Formula | C8H12N4O5 |
| Molecular Weight | 244.205 |
| Flash Point | 277.0±32.9 °C |
| Exact Mass | 244.080765 |
| PSA | 143.72000 |
| LogP | -1.99 |
| Vapour Pressure | 0.0±3.2 mmHg at 25°C |
| Index of Refraction | 1.823 |
| Water Solubility | 0.5-1.0 g/100 mL at 21 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H350 |
| Precautionary Statements | P201-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R45;R46;R22 |
| Safety Phrases | S53-S22-S36/37/39-S45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | XZ3017500 |
| HS Code | 2934999090 |
|
~71% 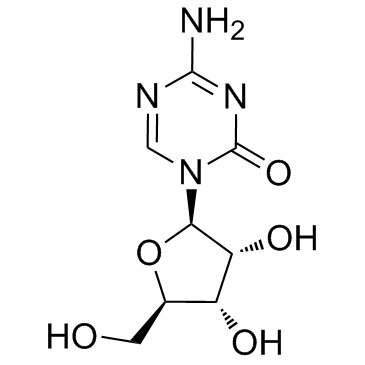
320-67-2 |
| Literature: ScinoPharm Taiwan Ltd. Patent: US2010/36112 A1, 2010 ; Location in patent: Page/Page column 7 ; |
|
~40% 
320-67-2 |
| Literature: Ionescu, Dumitru; Blumbergs, Peter Patent: US2004/186283 A1, 2004 ; Location in patent: Page 8 ; |
|
~75% 
320-67-2 |
| Literature: SICOR INC.; BIGATTI, Ettore; LUX, Giovanna; PAIOCCHI, Maurizio; GIOLITO, Andrea; TOSI, Simone Patent: WO2010/17374 A1, 2010 ; Location in patent: Page/Page column 18 ; |
|
~% 
320-67-2 |
| Literature: EP2371825 A1, ; Page/Page column 9 ; |
|
~% 
320-67-2 |
| Literature: EP2371825 A1, ; Page/Page column 9 ; |
|
~% 
320-67-2 |
| Literature: US2014/135490 A1, ; Page/Page column ; |
|
~75% 
320-67-2 |
| Literature: Vujjini, Satish Kumar; Varanasi, Ganesh; Arevelli, Srinivas; Kandala, Sreenatha Charyulu; Tirumalaraju, Satyanarayana Raju; Bandichhor, Rakeshwar; Kagga, Mukkanti; Cherukupally, Praveen Organic Process Research and Development, 2013 , vol. 17, # 2 p. 303 - 306 |
|
~% 
320-67-2 |
| Literature: WO2009/16617 A2, ; Page/Page column 15-16 ; |
|
~% 
320-67-2 |
| Literature: Journal of Pharmaceutical Sciences, , vol. 70, # 11 p. 1228 - 1232 |
| Precursor 9 | |
|---|---|
| DownStream 1 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |



![4-amino-1-[4-(hydroxymethyl)-7,7-dimethyl-3,6,8-trioxabicyclo[3.3.0]oct-2-yl]-1,3,5-triazin-2-one structure](https://image.chemsrc.com/caspic/217/65370-90-3.png)