254877-67-3
| Name | ataciguat |
|---|---|
| Synonyms |
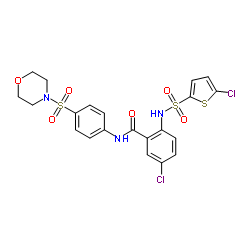
5-chloro-2-(5-chlorothiophene-2-sulfonylamino)-N-(4-(morpholine-4-sulfonyl)phenyl)benzamide
Benzamide, 5-chloro-2-[[(5-chloro-2-thienyl)sulfonyl]amino]-N-[4-(4-morpholinylsulfonyl)phenyl]- 5-Chloro-2-{[(5-chloro-2-thienyl)sulfonyl]amino}-N-[4-(4-morpholinylsulfonyl)phenyl]benzamide hmr 1766 ataciguat |
| Description | Ataciguat (HMR-1766) is a nitric oxide-independent soluble guanylate cyclase (sGC) activator. Ataciguat is able to activate the ferric heme-iron redox form of sGC that stimulate the production of cyclic GMP (cGMP). Ataciguat exhibits vasodilator effects[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
soluble guanylate cyclase[1] |
| In Vitro | Ataciguat (1-100 μM) induces the relaxation in aortic rings or coronary rings[2]. Ataciguat (0.1-10 μM; 30 min) increases NO production in HUVEC cells[2]. Ataciguat (1 nM-100 μM) induces concentration-dependent relaxations in sphincter of Oddi (SO) rings pre-contracted by Carbachol[3]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Molecular Formula | C21H19Cl2N3O6S3 |
| Molecular Weight | 576.493 |
| Exact Mass | 574.981323 |
| PSA | 166.88000 |
| LogP | 6.08 |
| Index of Refraction | 1.685 |
| Storage condition | 2-8°C |