2222-07-3
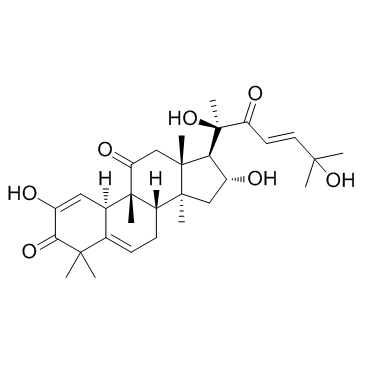
| Name | cucurbitacin I |
|---|---|
| Synonyms |
2,16,20,26-Tetrahydroxy-9,10,14-trimethyl-4,9-cyclo-9,10-secocholesta-2,5,24-triene-1,11,23-trione
CUCURBITACIN I Estra-1,5-diene-3,11-dione, 17-(1,6-dihydroxy-1,5-dimethyl-3-oxo-4-hexen-1-yl)-2,16-dihydroxy-4,4,9,14-tetramethyl- |
| Description | Cucurbitacin I is a natural selective inhibitor of JAK2/STAT3, with potent anti-cancer activity. |
|---|---|
| Related Catalog | |
| Target |
JAK2 STAT3 |
| In Vitro | Exposure of the COLO205 cells to Cucurbitacin I significantly decreases cell viability. The anticancer activity of Cucurbitacin I is accomplished by downregulating p-STAT3 and MMP-9 expression[1]. PE-induced cell enlargement and upregulation of ANF and β-MHC are significantly suppressed by pretreatment of the cardiomyocytes with Cucurbitacin I. Notably, Cucurbitacin I also impaires connective tissue growth factor (CTGF) and MAPK signaling, pro-hypertrophic factors, as well as TGF-β/Smad signaling, the important contributing factors to fibrosis[2]. Incubation of the Seax cell line with the Jak/Stat3 inhibitor Cucurbitacin I result in a time- and concentration-dependent decrease of P-Stat3 and Stat3. In freshly isolated Sz cells (n=3), Cucurbitacin I induces a concentration-dependent decrease in Stat3 expression whereas P-Stat3 is undetectable. Finally, incubation of freshly isolated Sz cells (n=4) with 30 μM Cucurbitacin I for 6 hours induces apoptosis in the large majority (73-91%) of tumor cells[3]. |
| In Vivo | No major side effects are noted throughout the study. It is shown that average tumor volumes at the end of the study are as follows: control, 616 mm3 (±130); CQ, 580 mm3 (±107); Cucurbitacin I, 346mm3 (±79); and combination, 220mm3 (±62). The differences in tumor volume between the Cucurbitacin I and control, combination and control, and combination and Cucurbitacin I arms are significant. Furthermore, combination-treated tumors exhibit a significantly lower average tumor weight at study termination than the control. Moreover, there was no effect on the body weights of mice[4]. |
| Animal Admin | Mice[4] BALB/c nude (nu/nu) female mice are used. U251 cells (5×106 cells in 50 μL of serum-free DMEM) are inoculated subcutaneously into the right flank of 5-week-old female mice after acclimatization for a week. Tumor growth is measured daily with calipers. When the tumors reach a mean volume of 90-120 mm3, animals are randomized into groups. In the first experiment, 16 mice are randomly assigned to Cucurbitacin I (1 mg/kg/day in 20% DMSO in PBS) or drug vehicle control (20% DMSO in PBS) and dosed intraperitoneally with 100 μL of vehicle or drug once daily for 18 days, whereas, in the second, 20 mice are assigned to four groups. Control animals receive 20% DMSO in PBS vehicle, whereas treated animals are injected with Cucurbitacin I (1 mg/kg/day) in 20% DMSO in PBS, CQ (25 mg/kg/day) in 20% DMSO in PBS, and Cucurbitacin I (1 mg/kg/day) plus CQ (25 mg/kg/day) in 20% DMSO in PBS and dosed intraperitoneally with 100 μL of vehicle or drug once daily for 15 days[4]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 716.9±60.0 °C at 760 mmHg |
| Melting Point | 148-150ºC |
| Molecular Formula | C30H42O7 |
| Molecular Weight | 514.650 |
| Flash Point | 401.3±29.4 °C |
| Exact Mass | 514.293030 |
| PSA | 132.13000 |
| LogP | 2.06 |
| Vapour Pressure | 0.0±5.2 mmHg at 25°C |
| Index of Refraction | 1.594 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | T+:Verytoxic; |
|---|---|
| Risk Phrases | R28 |
| Safety Phrases | S28-S36/37-S45 |
| RIDADR | UN 2811 |
| RTECS | RC6200000 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |