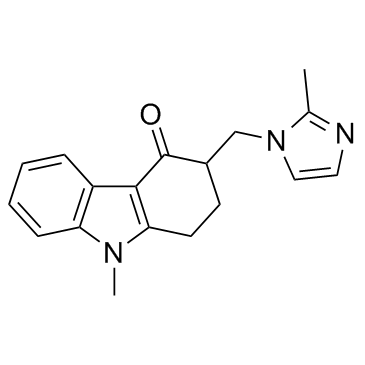
99614-01-4
| Name | ondansetron hydrochloride |
|---|---|
| Synonyms |
UNII:2999F27MAD
(R)-Ondansetron Hydrochloride Dihydrate 4H-CARBAZOL-4-ONE,1,2,3,9-TETRAHYDRO-9-METHYL-3-[(2-METHYL-1H-IMIDAZOL-1-YL)METHYL]-,HYDROCHLORIDE (1:1) 9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one LSUREMONOETHANOLAMID Ondansetron HCl 1,2,3,9-Tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one hydrochloride OEA,oleoylethanolamide N-(Hydroxyethyl)oleamide 9-Methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one hydrochloride [3H]-Oleoylethanolamide oleic acid ethanolamide ONDANSETRON HCL DIHYDRATE 9-methyl-3-[(2-methyl-1h-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4h-carbazol-4-onhydrochlorid Ondansetron HCl (Zofran) 4H-carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-, monohydrochloride oleic monoethanolamide Ondansetron (hydrochloride) ONDANSETRONHYDROCHLORIDE ONDANSETRON HCL DIHYDRATE IMP. E (EP): 1H-IMIDAZOLE, CRM STANDARD Ondansetron hydrochloride (Zofran) oleic acid-N-monoethanolamide N-(2-Hydroxyethyl)oleamide OEA Oleoylethanolamide [3H]-Ondansetron hydrochloride 4H-Carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-, hydrochloride (1:1) ODANSETRON HYDROCHLORIDE DIHYDRATE Ondansetron Hydrochloride Oleyl monoethanolamide OLEAMIDE MEA 9-méthyl-3-[(2-méthyl-1H-imidazol-1-yl)méthyl]-1,2,3,9-tétrahydro-4H-carbazol-4-one chlorhydrate Oleoylmonoethanolamide 9-Methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one hydrochloride (1:1) |
| Description | Ondansetron is a serotonin 5-HT3 receptor antagonist used mainly as anantiemetic (to treat nausea and vomiting), often following chemotherapy.Target: 5- HT ReceptorIC50 Value: in vitro: 5-HT evoked transient inward currents (EC50 = 3.4 microM; Hill coefficient = 1.8) that were blocked by the 5-HT3 receptor antagonist ondansetron (IC50 = 103 pM) [1]. The 5-HT3A receptor antagonist ondansetron (0.3 nM) reversibly inhibited the 5-HT (30 microM) signal by 70% and at 3 nM it abolished the response [2].in vivo: Acute ondansetron administration at the lowest dose (0.1 mg/kg, IP) tested had no effect, while other doses (0.33 and 1 mg/kg, IP) produced improvements in auditory gating [3]. Different doses of ondansetron were injected intraperitoneally (i.p.) at fixed times during the day to determine both the sublethal (TD50) and lethal (LD50) doses, which were, respectively, 3.7 +/- 0.6 mg/kg and 4.6 +/- 0.5 mg/kg [4]. ondansetron (0.25-1.0 mg/kg, subcutaneously) given before the challenge dose of ethanol (2.4 g/kg, intraperitoneally) injection, significantly and dose dependently attenuated the expression of sensitization. In addition, ondansetron (1.0 mg/kg, subcutaneously) given before ethanol injection on days 1, 4, 7, and 10 significantly blocked the development (days 1, 4, 7, and 10), and expression (day 15) of sensitization to the locomotor stimulant effect of ethanol injection [5]. Toxicity: Ondansetron may be safe in lower doses used to prevent nausea and vomiting in radiation treatment or postoperatively. However, as there is a report that a lower dose of ondansetron prolonged the QT interval in healthy volunteers, this needs to be clarified by the FDA [6]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.27g/cm3 |
|---|---|
| Boiling Point | 546ºC at 760mmHg |
| Melting Point | 178.5-179.5ºC |
| Molecular Formula | C18H20ClN3O |
| Molecular Weight | 329.824 |
| Flash Point | 284ºC |
| Exact Mass | 329.129486 |
| PSA | 39.82000 |
| LogP | 3.93050 |
| Storage condition | −20°C |
| Water Solubility | H2O: >5 mg/mL |
| Hazard Codes | T: Toxic; |
|---|---|
| Risk Phrases | R25 |
| Safety Phrases | 45-37/39-26 |
| RIDADR | UN 2811 6 |
| WGK Germany | 3 |
| RTECS | FE6375500 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| HS Code | 29339900 |
|
~90% 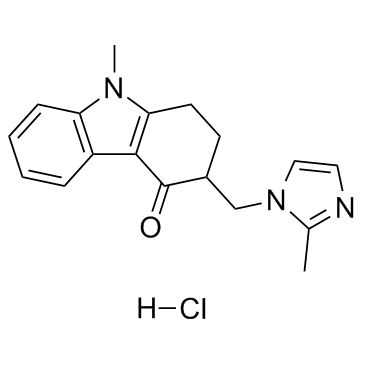
99614-01-4 |
| Literature: WO2005/37822 A1, ; Page/Page column 12 ; |
|
~78% 
99614-01-4 |
| Literature: WO2005/37822 A1, ; Page/Page column 12 ; |
|
~93% 
99614-01-4 |
| Literature: YUHAN CORPORATION Patent: WO2005/37822 A1, 2005 ; Location in patent: Page/Page column 12 ; |
| Precursor 4 | |
|---|---|
| DownStream 2 | |