TC-G 1004
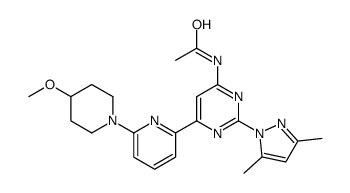
TC-G 1004 structure
|
Common Name | TC-G 1004 | ||
|---|---|---|---|---|
| CAS Number | 1061747-72-5 | Molecular Weight | 421.49500 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C22H27N7O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of TC-G 1004TC-G 1004 (compound 16j) is an orally active A2A adenosine receptor antagonist, with Ki values of 0.44 nM and 80 nM for hA2A and hA1, respectively[1]. |
| Name | N-{2-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-[6-(4-methoxy-1-piperidinyl )-2-pyridinyl]-4-pyrimidinyl}acetamide |
|---|---|
| Synonym | More Synonyms |
| Description | TC-G 1004 (compound 16j) is an orally active A2A adenosine receptor antagonist, with Ki values of 0.44 nM and 80 nM for hA2A and hA1, respectively[1]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 0.44 nM (hA2A), 80 nM (hA1)[1]. |
| In Vitro | TC-G 1004 (compound 16j) is a highly potent A2A antagonist at both the human and rat receptors with greater than 100× selectivity over hA1[1]. |
| In Vivo | TC-G 1004 (compound 16j, 3 mg/kg) displays good in vivo oral activity in rat models for Parkinson’s disease and were advanced to preclinical development[1]. TC-G 1004 (compound 16j) dose-dependently potentiates L-dopa induced rotations with an MED of 3 mg/kg when dosed orally[1]. |
| References |
| Molecular Formula | C22H27N7O2 |
|---|---|
| Molecular Weight | 421.49500 |
| Exact Mass | 421.22300 |
| PSA | 101.55000 |
| LogP | 3.62920 |
|
~41% 
TC-G 1004 CAS#:1061747-72-5 |
| Literature: NEUROCRINE BIOSCIENCES, INC. Patent: WO2008/116185 A2, 2008 ; Location in patent: Page/Page column 44-45 ; WO 2008/116185 A2 |
|
~22% 
TC-G 1004 CAS#:1061747-72-5 |
| Literature: Zhang, Xiaohu; Tellew, John E.; Luo, Zhiyong; Moorjani, Manisha; Lin, Emily; Lanier, Marion C.; Chen, Yongsheng; Williams, John P.; Saunders, John; Lechner, Sandra M.; Markison, Stacy; Joswig, Tanya; Petroski, Robert; Piercey, Jaime; Kargo, William; Malany, Siobhan; Santos, Mark; Gross, Raymond S.; Wen, Jenny; Jalali, Kayvon; O'Brien, Zhihong; Stotz, Carol E.; Crespo, Maria I.; Diaz, Jose-Luis; Slee, Deborah H. Journal of Medicinal Chemistry, 2008 , vol. 51, # 22 p. 7099 - 7110 |
| cs-1344 |
![2-(3,5-dimethyl-pyrazol-1-yl)-6-(4-methoxy-3,4,5,6-tetrahydro-2H-[1,2']bipyridinyl-6'-yl)-pyrimidin-4-ylamine trifluoroacetate structure](https://image.chemsrc.com/caspic/386/1061747-75-8.png)


![N-[6-(6-chloropyridin-2-yl)-2-(3,5-dimethyl-pyrazol-1-yl)-pyrimidin-4-yl]-acetamide structure](https://image.chemsrc.com/caspic/348/1061750-08-0.png)