Isocytosine
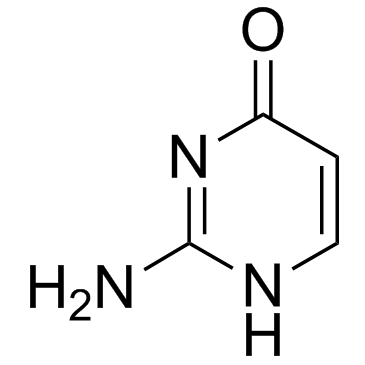
Isocytosine structure
|
Common Name | Isocytosine | ||
|---|---|---|---|---|
| CAS Number | 108-53-2 | Molecular Weight | 111.102 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 393.6±34.0 °C at 760 mmHg | |
| Molecular Formula | C4H5N3O | Melting Point | 275°C | |
| MSDS | Chinese USA | Flash Point | 191.9±25.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of IsocytosineIsocytosine is a non-natural nucleobase and an isomer of cytosine. It is used in combination with Isoguanine in studies of unnatural nucleic acid analogues of the normal base pairs in DNA and used as a nucleobase of hachimoji RNA[1][2]. |
| Name | 2-amino-4-hydroxypyrimidine |
|---|---|
| Synonym | More Synonyms |
| Description | Isocytosine is a non-natural nucleobase and an isomer of cytosine. It is used in combination with Isoguanine in studies of unnatural nucleic acid analogues of the normal base pairs in DNA and used as a nucleobase of hachimoji RNA[1][2]. |
|---|---|
| Related Catalog | |
| References |
[1]. "Isocytosine". Molecule of the Week. American Chemical Society. Retrieved November 1, 2012. |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 393.6±34.0 °C at 760 mmHg |
| Melting Point | 275°C |
| Molecular Formula | C4H5N3O |
| Molecular Weight | 111.102 |
| Flash Point | 191.9±25.7 °C |
| Exact Mass | 111.043259 |
| PSA | 72.03000 |
| LogP | -1.01 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.668 |
| Storage condition | Room temperature. |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22;R36 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933599090 |
| Precursor 10 | |
|---|---|
| DownStream 8 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Lead optimization of isocytosine-derived xanthine oxidase inhibitors.
Bioorg. Med. Chem. Lett. 23(3) , 834-8, (2013) We report our attempts at improving the oral efficacy of low-nanomolar inhibitors of xanthine oxidase from isocytosine series through chemical modifications. Our lead compound had earlier shown good i... |
|
|
Isocytosine-based inhibitors of xanthine oxidase: Design, synthesis, SAR, PK and in vivo efficacy in rat model of hyperuricemia
Bioorg. Med. Chem. Lett. 22(24) , 7543-6, (2012) Structure-activity relationship studies were carried out for lead generation following structure-guided design approach from an isocytosine scaffold identified earlier for xanthine oxidase inhibition.... |
|
|
Indirect photochemical transformations of acyclovir and penciclovir in aquatic environments increase ecological risk.
Environ. Toxicol. Chem. 35 , 584-92, (2016) Acyclovir and penciclovir, 2 antiviral drugs, are increasingly detected in aquatic environments. The present study explores the natural photochemical transformation mechanisms and fate of these drugs,... |
| 4(1H)-Pyrimidinone, 2-amino- (9CI) |
| 2-amino-4-oxo-3,4-dihydropyrimidine |
| 4(3H)-pyrimidinone, 2-amino- |
| 2-amino-3H-pyrimidin-4-one |
| Isocytosine |
| 2-Amino-4-pyrimidone |
| 2-Aminopyrimidin-4-ol |
| 2-amino-4-hydroxypyrimine |
| 2-Amino-4-hydroxypyrimidine |
| 4-Hydroxy-2-aminopyrimidine |
| 2-amino-1H-pyrimidin-6-one |
| MFCD00057557 |
| 2-Amino-4-hydroxypyrimidine,2-Aminouracil |
| 2-Aminopyrimidin-4(1H)-one |
| Iso Cytosine |
| 2-Amino-4(1H)-pyrimidinone |
| 2-Aminopyrimidin-4(3H)-one |
| 2-Amino-6-hydroxpyrimidine |
| EINECS 203-592-0 |
| 2-aminouracil |
| 4-Pyrimidinol, 2-amino- |
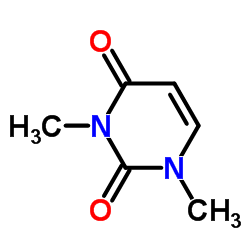 CAS#:874-14-6
CAS#:874-14-6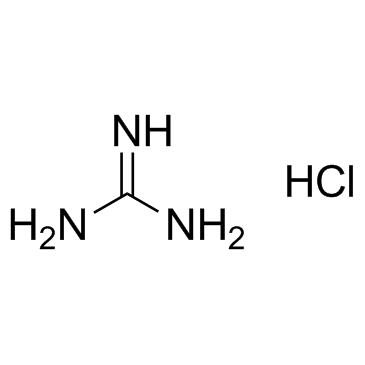 CAS#:50-01-1
CAS#:50-01-1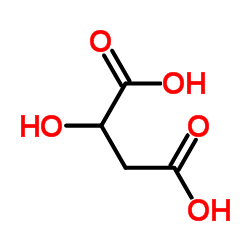 CAS#:617-48-1
CAS#:617-48-1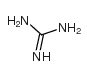 CAS#:113-00-8
CAS#:113-00-8 CAS#:109-12-6
CAS#:109-12-6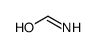 CAS#:77287-34-4
CAS#:77287-34-4![Imidazo[1,2-a]pyrimidin-5(1H)-one Structure](https://image.chemsrc.com/caspic/369/55662-68-5.png) CAS#:55662-68-5
CAS#:55662-68-5 CAS#:25957-58-8
CAS#:25957-58-8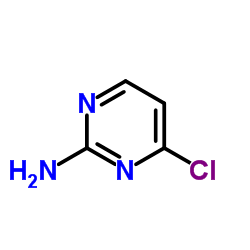 CAS#:3993-78-0
CAS#:3993-78-0 CAS#:5751-20-2
CAS#:5751-20-2 CAS#:66-22-8
CAS#:66-22-8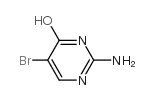 CAS#:61937-71-1
CAS#:61937-71-1 CAS#:55662-33-4
CAS#:55662-33-4 CAS#:7254-29-7
CAS#:7254-29-7 CAS#:629645-53-0
CAS#:629645-53-0
