Gemcitabine HCl
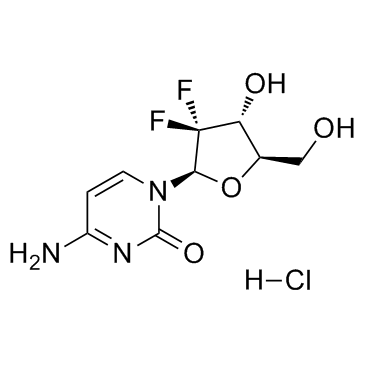
Gemcitabine HCl structure
|
Common Name | Gemcitabine HCl | ||
|---|---|---|---|---|
| CAS Number | 122111-03-9 | Molecular Weight | 299.659 | |
| Density | N/A | Boiling Point | 482.7ºC at 760 mmHg | |
| Molecular Formula | C9H12ClF2N3O4 | Melting Point | >250°C dec. | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of Gemcitabine HClGemcitabine hydrochloride is a DNA synthesis inhibitor with IC50s of 37.6, 42.9, 92.7, 89.3 and 131.4 nM in BxPC-3, Mia Paca-2, PANC-1, PL-45 and AsPC-1 cells, respectively. |
| Name | gemcitabine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Gemcitabine hydrochloride is a DNA synthesis inhibitor with IC50s of 37.6, 42.9, 92.7, 89.3 and 131.4 nM in BxPC-3, Mia Paca-2, PANC-1, PL-45 and AsPC-1 cells, respectively. |
|---|---|
| Related Catalog | |
| Target |
DNA synthesis[1] |
| In Vitro | MTS assay demonstrates that Gemcitabine at 15 nM, indole-3-carbinol (I3C) at 50 μM and the combination does not affect hTERT-HPNE cell viability. However, treatment with Gemcitabine at 15 nM, I3C at 50 μM and the combination results in 31%, 19% and 72% cell death of BxPC-3 cells, respectively[1]. |
| In Vivo | The aim of study is to formulate PLGA nanoparticles (NPs) of Gemcitabine Hydrochloride (Gemcitabine HCl) for enhanced oral bioavailability via absorption through M cells of Peyer’s patches. Gemcitabine HCl is available as i.v. infusion due to its short half life (8-17 min), rapid metabolism and limited tumor uptake. Gemcitabine loaded PLGA NPs shows 21.47-fold increase in relative bioavailability in comparison to plain drug solution after oral delivery[2]. After i.v. injection of Gemcitabine at doses of 50, 100, and 120, and 300 mg/kg, the highest dose caused considerable body weight loss (p<0.05 at all the time points evaluated, starting from day 3 of first injection) compared with that of the untreated group and complete mortality, whereas 120 mg/kg is determined as the lethal dose 10% (LD10) and 100 mg/kg is considered as the maximal tolerated dose, which does not cause any mortality and a minimal body weight loss[3]. |
| Cell Assay | Cells (the human pancreatic cell lines, Mia PaCa-2, BxPC-3, AsPC-1, PANC-1, PL-45, and normal pancreatic ductal epithelial cells, hTERT-HPNE cells) are seeded into 96-well plates (3000 cells/well) in triplicate. After overnight incubation, the medium is changed and cells are treated with I3C and/or NBMPR for 24 h. The medium is changed again and cells are cultured in medium containing different concentrations of Gemcitabine in the presence or absence of the same concentrations of I3C and/or NBMPR for 48 h. The cells are then subjected to CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS). Absorbance at 490 nm is measured 2 h after the addition of 20 μL of MTS reagent/well[1]. The in vitro cytotoxicity of Gemcitabine HCl loaded NPs on Caco-2 cells are performed for 6 h to check the toxicity of NPs during the transport/permeability studies and antiproliferative effect on K562 cells is evaluated for 48 h using the MTT assay. The cells are cultured in 96-well plates at a seeding density of 1.0×104 cells/well for 48 h. Experiments are initiated by replacing the culture medium in each well with 150 μL of sample solutions (0.1, 1, 10, 100 μg/mL) at 37°C in the CO2 incubator. After 4, 24 and 48 h of incubation, the medium is removed and 150 μL of MTT reagent (1 mg/mL) in the serum-free medium is added to each well. The plates are then incubated at 37°C for another 4 h. At the end of the incubation period, the medium is removed and the intracellular formazan is solubilized with 150 μL DMSO and quantified by reading the absorbance at 590 nm on a micro-plate multi-detection instrument, SpectraMax M2 with Soft Max Pro. The medium treated cells serve as controls. Percentage cell viability is calculated based on the absorbance measured relative to the absorbance of cells exposed to the negative control[2]. |
| Animal Admin | Rats[2] Three groups of male Wistar rats (n=6) are subjected to single oral dose bioavailability study. The formulations are administered orally with the aid of a syringe and infant feeding tube. The 1st group is given distilled water, the 2nd group is given a solution of Gemcitabine HCl in distilled water, and the third group received Gemcitabine HCl loaded NPs at a dose of 10 mg/kg. Blood samples (0.3 mL) are drawn by retro-orbital venous plexus puncture with the aid of capillary tubes at 0.5, 1, 2, 4, 24, 48, 72 h post oral dose. The samples are collected in heparinised Eppendorf tubes containing 10 μM tetrahydrouridine, centrifuged at 3400 rpm for 15 min and plasma is collected. To this, 200 μL of Acetonitrile is added and vortexed for 5 min followed by centrifugation at 5000 rpm for 15 min. The organic phase is separated and evaporated under reduced pressure in a vacuum oven. The residue is dissolved in mobile phase (0.15 mL), vortexed for 1 min followed by centrifugation at 13,000 rpm for 5 min. Then 20 μL of supernatant is injected into the HPLC column and analyzed by HPLC. Mice[3] DBA/2 mice (5-6 weeks old), weighing approximately 15 to 18 g, are used for the study. The mice are provided with standard mouse food and water ad libitum. The L1210 wt leukemia cells are maintained in vitro, and they are injected intravenously (1×105) into the mice, to develop a systemic metastatic leukemia model. The mice are divided into six groups of seven to eight mice each: untreated, treated with squalene nanoparticles, treated with 100 mg/kg Gemcitabine, treated with 20 mg/kg equivalent SQgem nanoassemblies in Gemcitabine, treated with 100 mg/kg cytarabine, and treated with 100 mg/kg cytarabine every day for 5 days. After injection of leukemia cells (day 0), all groups of mice received the treatment by i.v. injection on days 1, 5, 9, and 14 (i.e., days after injecting leukemia cells), with the exception of the untreated group and the group treated with cytarabine daily by the i.v. route. The mice are monitored regularly for weight differences and survival. |
| References |
| Boiling Point | 482.7ºC at 760 mmHg |
|---|---|
| Melting Point | >250°C dec. |
| Molecular Formula | C9H12ClF2N3O4 |
| Molecular Weight | 299.659 |
| Exact Mass | 299.048431 |
| PSA | 110.60000 |
| LogP | 0.09460 |
| Vapour Pressure | 2.41E-11mmHg at 25°C |
| Storage condition | Desiccate at +4°C |
|
Section I.Chemical Product and Company Identification Chemical Name Gemcitabine Hydrochloride Portland OR SynonymCytidine, 2'-deoxy-2',2'-difluoro-, hydrochloride (1:1) (CA INDEX NAME) Chemical FormulaC9H11F2N3O4 · HCl CAS Number122111-03-9
Section II.Composition and Information on Ingredients Toxicology Data Chemical NameCAS Number Percent (%)TLV/PEL Min. 98.0 Not available.Rat LD50 (intravenous) 236 mg/kg Gemcitabine Hydrochloride122111-03-9 (HPLC,N)Mouse LD50 (intravenous) 500 mg/kg Section III. Hazards Identification Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the Acute Health Effects eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound. CARCINOGENIC EFFECTS : Not available. Chronic Health Effects MUTAGENIC EFFECTS : Not available. TERATOGENIC EFFECTS : Not available. DEVELOPMENTAL TOXICITY: Reproductive effects. Mouse TDLo Intravenous 15 mg/kg, female 6-15 days of pregnancy Toxic Effects: Maternal Effects - Parturition Effects on Fertility - Post-implantation mortality Effects on Fertility - Litter size Mouse TDLo Intravenous 15 mg/kg, female 6-15 days of pregnancy Toxic Effects: Maternal Effects - Other effects Effects on Embryo or Fetus - Fetotoxicity Effects on Embryo or Fetus - Fetal death Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions. Section IV.First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical attention. Skin ContactIn case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention. If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or Inhalation waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not improve. INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat. Ingestion Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material was ingested; the absence of such signs, however, is not conclusive. Section V.Fire and Explosion Data Not available. May be combustible at high temperature.Auto-Ignition Flammability Flash PointsFlammable LimitsNot available. Not available. These products are toxic carbon oxides (CO, CO2), nitrogen oxides (NO, NO2), halogenated compounds. Combustion Products WARNING: Highly toxic HCl gas is produced during combustion. WARNING: Highly toxic HF gas is produced during combustion. Fire Hazards Not available. Continued on Next Page Gemcitabine Hydrochloride Explosion HazardsRisks of explosion of the product in presence of mechanical impact: Not available. Risks of explosion of the product in presence of static discharge: Not available. Fire Fighting MediaSMALL FIRE: Use DRY chemical powder. LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet. and Instructions Consult with local fire authorities before attempting large scale fire-fighting operations. Section VI.Accidental Release Measures Spill CleanupIrritating material. This material is a reproductive effector. Use a shovel to put the material into a convenient waste disposal container. Consult federal, state, and/or local authorities for Instructions assistance on disposal. Section VII. Handling and Storage IRRITANT. REPRODUCTIVE EFFECTOR. Keep away from heat. Mechanical exhaust required. When not in use, tightly Handling and Storage seal the container and store in a dry, cool place. Avoid excessive heat and light. Do not breathe dust. Information Always store away from incompatible compounds such as oxidizing agents. Section VIII. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSplash goggles. Lab coat. Dust respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent. Not available. Exposure Limits Section IX. Physical and Chemical Properties Physical state @ 20°CSolid. (White crystalline powder.)SolubilitySoluble in hot water. Not available. Specific Gravity Molecular Weight299.66Partition CoefficientNot available. Boiling PointNot available.Not applicable. Vapor Pressure Melting Point287 to 292°C (548.6 to 557.6°F) (dec.)Vapor DensityNot available. Not available.Not available. Refractive IndexVolatility Critical TemperatureNot available.OdorNot available. Not available.Not available. ViscosityTaste Section X.Stability and Reactivity Data This material is stable if stored under proper conditions. (See Section VII for instructions) Stability Conditions of InstabilityAvoid excessive heat and light. IncompatibilitiesReactive with oxidizing agents. Section XI. Toxicological Information RTECS NumberHA3840000 Eye Contact. Ingestion. Inhalation. Routes of Exposure Rat LD50 (intravenous) 236 mg/kg Toxicity Data Mouse LD50 (intravenous) 500 mg/kg CARCINOGENIC EFFECTS : Not available. Chronic Toxic Effects MUTAGENIC EFFECTS : Not available. TERATOGENIC EFFECTS : Not available. DEVELOPMENTAL TOXICITY: Reproductive effects. Mouse TDLo Intravenous 15 mg/kg, female 6-15 days of pregnancy Toxic Effects: Maternal Effects - Parturition Effects on Fertility - Post-implantation mortality Effects on Fertility - Litter size Mouse TDLo Intravenous 15 mg/kg, female 6-15 days of pregnancy Toxic Effects: Maternal Effects - Other effects Effects on Embryo or Fetus - Fetotoxicity Effects on Embryo or Fetus - Fetal death Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions. Continued on Next Page Gemcitabine Hydrochloride Acute Toxic EffectsIrritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound. Section XII.Ecological Information Not available. Ecotoxicity Environmental FateNot available. Section XIII. Disposal Considerations Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a Waste Disposal combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when disposing of the substance. Section XIV. Transport Information DOT ClassificationNot a DOT controlled material (United States). PIN NumberNot applicable. Proper Shipping NameNot applicable. Packing Group (PG)Not applicable. DOT Pictograms Section XV. Other Regulatory Information and Pictograms TSCA Chemical InventoryThis product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40 CFR 720.36 (C) for those products not on the inventory list: (EPA) (i) These products are supplied solely for use in research and development by or under the supervision of a technically qualified individual as defined in 40 CFR 720.0 et sec. (ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be supplied on an MSDS sheet. WHMIS ClassificationNot available. (Canada) EINECS Number (EEC) 601-823-3 EEC Risk StatementsR36/37/38- Irritating to eyes, respiratory system and skin. R60- May impair fertility. R61- May cause harm to the unborn child. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H360 |
| Precautionary Statements | P201-P280-P308 + P313 |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R21 |
| Safety Phrases | 25-26-36/37-53 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2934999090 |
|
~89% 
Gemcitabine HCl CAS#:122111-03-9 |
| Literature: Hanmi Pharm. Co.,Ltd Patent: US2007/249818 A1, 2007 ; Location in patent: Page/Page column 10 ; |
|
~51% 
Gemcitabine HCl CAS#:122111-03-9 |
| Literature: KANNAN, Vishwanath; KASH, Vishwanathan Patent: WO2010/29574 A2, 2010 ; Location in patent: Page/Page column 8-9 ; |
|
~% 
Gemcitabine HCl CAS#:122111-03-9 |
| Literature: WO2007/117760 A2, ; Page/Page column 21 ; |
|
~% 
Gemcitabine HCl CAS#:122111-03-9 |
| Literature: WO2005/95430 A1, ; Page/Page column 18-20 ; |
|
~% 
Gemcitabine HCl CAS#:122111-03-9 |
| Literature: WO2007/49294 A1, ; Page/Page column 10-11 ; |
|
~% 
Gemcitabine HCl CAS#:122111-03-9 |
| Literature: WO2007/112473 A1, ; Page/Page column 8-9 ; |
| Precursor 6 | |
|---|---|
| DownStream 4 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Quantitative proteomics of the yeast Hsp70/Hsp90 interactomes during DNA damage reveal chaperone-dependent regulation of ribonucleotide reductase.
J. Proteomics 112 , 285-300, (2015) The highly conserved molecular chaperones Hsp90 and Hsp70 are indispensible for folding and maturation of a significant fraction of the proteome, including many proteins involved in signal transductio... |
|
|
Co-delivery of paclitaxel and gemcitabine via CD44-targeting nanocarriers as a prodrug with synergistic antitumor activity against human biliary cancer.
Biomaterials 53 , 763-74, (2015) Multi-drug delivery focuses on different signaling pathways in cancer cells that have synergistic anti-proliferative effects. In this study, we developed multi-prodrug nanocarriers (MPDNCs) consisting... |
|
|
Systemic delivery of microencapsulated 3-bromopyruvate for the therapy of pancreatic cancer.
Clin. Cancer Res. 20(24) , 6406-17, (2014) This study characterized the therapeutic efficacy of a systemically administered formulation of 3-bromopyruvate (3-BrPA), microencapsulated in a complex with β-cyclodextrin (β-CD), using an orthotopic... |
| dFdC,Gemzar,Lilly) |
| MFCD01735988 |
| dFdC Gemzar (Lilly) LY-188011 dFdC dFdCyd |
| 4-Amino-1-[(2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one hydrochloride |
| Gemcitabine hydrochloride |
| 2'-Deoxy-2',2'-difluorocytidine |
| 4-Amino-1-[(2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydro-2-furanyl]-2(1H)-pyrimidinone hydrochloride |
| Cytidine, 2'-deoxy-2',2'-difluoro-, hydrochloride (1:1) |
| 2'-Deoxy-2',2'-difluorocytidine hydrochloride (1:1) |
| Gemcitabine HCl |
| Gemcitabine HCl(TABINES) |
| Gemcitabine (Hydrochloride) |


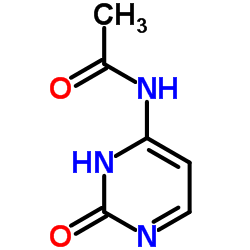
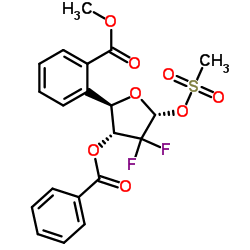
 CAS#:143157-27-1
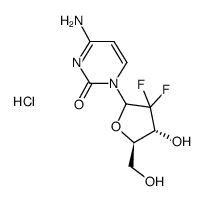
CAS#:143157-27-1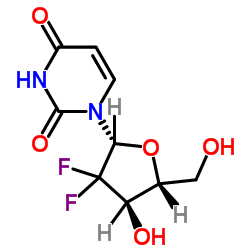 CAS#:114248-23-6
CAS#:114248-23-6![2'-Deoxy-3',5'-bis-O-[dimethyl(2-methyl-2-propanyl)silyl]-2',2'-d ifluorocytidine structure](https://image.chemsrc.com/caspic/046/688009-09-8.png) CAS#:688009-09-8
CAS#:688009-09-8
