cabazitaxel
Modify Date: 2024-01-02 11:42:14
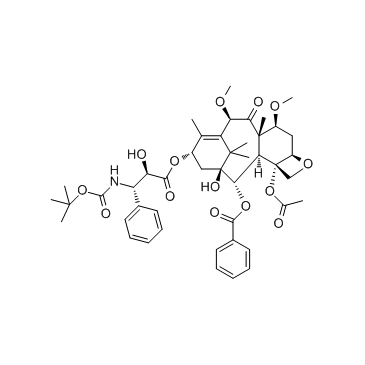
cabazitaxel structure
|
Common Name | cabazitaxel | ||
|---|---|---|---|---|
| CAS Number | 183133-96-2 | Molecular Weight | 835.932 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 870.7±65.0 °C at 760 mmHg | |
| Molecular Formula | C45H57NO14 | Melting Point | 180 °C | |
| MSDS | N/A | Flash Point | 480.4±34.3 °C | |
Use of cabazitaxelCabazitaxel is a semi-synthetic derivative of the natural taxoid 10-deacetylbaccatin III with potential antineoplastic activity. |
| Name | cabazitaxel |
|---|---|
| Synonym | More Synonyms |
| Description | Cabazitaxel is a semi-synthetic derivative of the natural taxoid 10-deacetylbaccatin III with potential antineoplastic activity. |
|---|---|
| Related Catalog | |
| In Vitro | The cytotoxicity of cabazitaxel (100 μg/mL) on 4T1 cells without irradiation is 70.8%. Cabazitaxel (100 μg/mL) exhibits a concentration-dependent antiproliferation effect, with the antiproliferative activity of 56.2%[1]. |
| In Vivo | Cabazitaxel (10 mg/kg, i.v.) has certain toxicity to liver and kidney but it can be avoided by integrated into Ans. The body weights of mice treated with AN-ICG-CBX and AN-CBX have a slightly decrease, while body weights of the free CBX group significantly decrease compared to the control group[1]. |
| Cell Assay | The cytotoxicity of CBX-loaded ANs and free Cabazitaxel (CBX) is evaluated with MTT assay. Cells are seeded onto a 96-well plate at a density of 3000 cells per well and cultured for 24 h. CBX-loaded ANs and free CBX are diluted to predetermined concentrations with PBS and added into each well. Blank AN, AN-ICG and free CBX solvent (a mixture of Tween-80 and anhydrous alcohol) are added as well to different final concentrations. The incubation continued for another 48 hours. 20 µL MTT solutions (5 mg/mL in PBS) are added into each well and cells are incubated for another 4 hours under 37°C. Subsequently the medium is removed and 150 µL dimethyl sulphoxide (DMSO) is added to dissolve the purple formazan salt crystals. Then the absorbance is measured by a microplate reader at 490 nm. The cells treated with medium are evaluated as controls. |
| Animal Admin | To evaluate the antitumor efficiency of the combined chemotherapy and PTT in vivo, mice bearing 4T1 tumor are randomLy divided into 6 treatment groups (n=5). Treatment begin when the tumors reached 50 mm3-100 mm3. The mice are intravenously injected with saline, AN-ICG, free Cabazitaxel (CBX), AN-CBX and AN-ICG-CBX (ICG 2 mg/kg, CBX 10 mg/kg). 8 hours later, the groups injected with AN-ICG and AN-ICG-CBX is irradiated by the 808 nm laser (0.8 W/cm2, 5 min). The length and width of every tumor are measured by a caliper every other day. The formula (volume (mm3) =1/2 × length × width2) is used to calculate the tumor volume. The body weights of these mice are recorded every two days using an electronic balance as well. At the end of the antitumor study, the 4T1 tumor bearing mice are sacrificed to collect the tumors and major organs. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 870.7±65.0 °C at 760 mmHg |
| Melting Point | 180 °C |
| Molecular Formula | C45H57NO14 |
| Molecular Weight | 835.932 |
| Flash Point | 480.4±34.3 °C |
| Exact Mass | 835.377930 |
| PSA | 202.45000 |
| LogP | 7.55 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.592 |
| Storage condition | -20°C |
| HS Code | 2942000000 |
|---|
| Benzenepropanoic acid, β-[[(1,1-dimethylethoxy)carbonyl]amino]-α-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-h ydroxy-4,6-dimethoxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)- |
| Jevtana |
| (2α,5β,7β,10β,13α)-4-Acetoxy-1-hydroxy-13-{[(2R,3S)-2-hydroxy-3-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)-3-phenylpropanoyl]oxy}-7,10-dimethoxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoa 
te |
| Cabazitaxelum |
| cabazitaxel |
| Taxoid XRP6258 |
| TXD258 |
| XRP-6258 |
| (1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-(acetyloxy)-15-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-3-phenylpropanoyl]oxy}-1-hydroxy-9,12-dimethoxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04'7]heptadec-13-en-2-ylbenzoate |
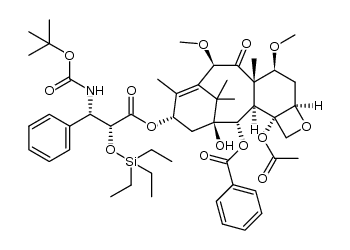 CAS#:1380584-07-5
CAS#:1380584-07-5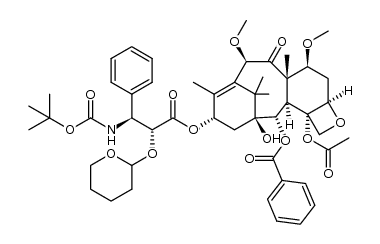 CAS#:1372883-35-6
CAS#:1372883-35-6 CAS#:1352816-81-9
CAS#:1352816-81-9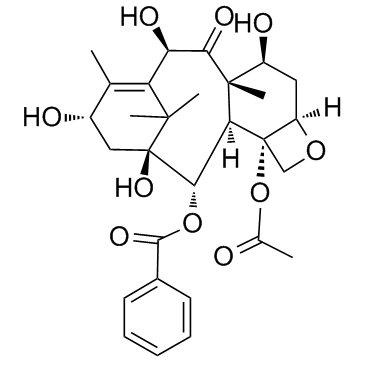 CAS#:32981-86-5
CAS#:32981-86-5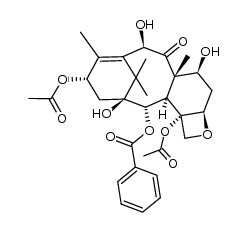 CAS#:110258-96-3
CAS#:110258-96-3
