Silymarin
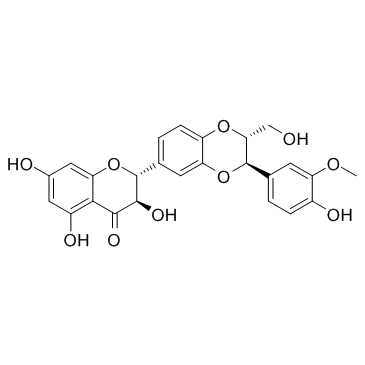
Silymarin structure
|
Common Name | Silymarin | ||
|---|---|---|---|---|
| CAS Number | 22888-70-6 | Molecular Weight | 482.436 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 793.0±60.0 °C at 760 mmHg | |
| Molecular Formula | C25H22O10 | Melting Point | 164-174°C | |
| MSDS | Chinese USA | Flash Point | 274.5±26.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of SilymarinSilibinin, an effective anti-cancer and chemopreventive agent, has been shown to exert multiple effects on cancer cells, including inhibition of both cell proliferation and migration.IC50 value:Target: anticancerin vitro: silibinin significantly induced the expression of the non-steroidal anti-inflammatory drug-activated gene-1 (NAG-1) in both p53 wild-type and p53-null cancer cell lines, suggesting that silibinin-induced NAG-1 up-regulation is p53-independent manner.Silibinin up-regulates early growth response-1 (EGR-1) expression [1]. silibinin induced cell death in human breast cancer cell lines MCF7 and MDA-MB-231. Silibinininduced cell death was attenuated by antioxidants, N-acetylcysteine (NAC) and Trolox, suggesting that the effect of silibinin was dependent on generation of reactive oxygen species (ROS) [2]. SIL treatment resulted in a dose- and time-dependent inhibition of HCC cell viability, SIL exhibited strong antitumor activity, as evidenced not only by reductions in tumor cell adhesion, migration, intracellular glutathione (GSH) levels and total antioxidant capability (T-AOC) but also by increases in the apoptotic index, caspase3 activity, and reactive oxygen species (ROS). SIL treatment decreased the expression of the Notch1 intracellular domain (NICD), RBP-Jκ, and Hes1 proteins, upregulated the apoptosis pathway-related protein Bax, and downregulated Bcl2, survivin, and cyclin D1. Notch1 siRNA (in vitro) or DAPT (a known Notch1 inhibitor, in vivo) further enhanced the antitumor activity of SIL, and recombinant Jagged1 protein (a known Notch ligand in vitro) attenuated the antitumor activity of SIL [3].in vivo: Topical application of silibinin at the dose of 9 mg/mouse effectively suppressed oxidative stress and deregulated activation of inflammatory mediators and tumorigenesis[4]. The kidney cortex of vehicle-treated control OVE26 mice displayed greater Nox4 expression and twice as much superoxide production than cortex of silybin-treated mice. The glomeruli of control OVE26 mice displayed 35% podocyte drop out that was not present in the silybin-treated mice [5]. |
| Name | Silibinin |
|---|---|
| Synonym | More Synonyms |
| Description | Silibinin, an effective anti-cancer and chemopreventive agent, has been shown to exert multiple effects on cancer cells, including inhibition of both cell proliferation and migration.IC50 value:Target: anticancerin vitro: silibinin significantly induced the expression of the non-steroidal anti-inflammatory drug-activated gene-1 (NAG-1) in both p53 wild-type and p53-null cancer cell lines, suggesting that silibinin-induced NAG-1 up-regulation is p53-independent manner.Silibinin up-regulates early growth response-1 (EGR-1) expression [1]. silibinin induced cell death in human breast cancer cell lines MCF7 and MDA-MB-231. Silibinininduced cell death was attenuated by antioxidants, N-acetylcysteine (NAC) and Trolox, suggesting that the effect of silibinin was dependent on generation of reactive oxygen species (ROS) [2]. SIL treatment resulted in a dose- and time-dependent inhibition of HCC cell viability, SIL exhibited strong antitumor activity, as evidenced not only by reductions in tumor cell adhesion, migration, intracellular glutathione (GSH) levels and total antioxidant capability (T-AOC) but also by increases in the apoptotic index, caspase3 activity, and reactive oxygen species (ROS). SIL treatment decreased the expression of the Notch1 intracellular domain (NICD), RBP-Jκ, and Hes1 proteins, upregulated the apoptosis pathway-related protein Bax, and downregulated Bcl2, survivin, and cyclin D1. Notch1 siRNA (in vitro) or DAPT (a known Notch1 inhibitor, in vivo) further enhanced the antitumor activity of SIL, and recombinant Jagged1 protein (a known Notch ligand in vitro) attenuated the antitumor activity of SIL [3].in vivo: Topical application of silibinin at the dose of 9 mg/mouse effectively suppressed oxidative stress and deregulated activation of inflammatory mediators and tumorigenesis[4]. The kidney cortex of vehicle-treated control OVE26 mice displayed greater Nox4 expression and twice as much superoxide production than cortex of silybin-treated mice. The glomeruli of control OVE26 mice displayed 35% podocyte drop out that was not present in the silybin-treated mice [5]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 793.0±60.0 °C at 760 mmHg |
| Melting Point | 164-174°C |
| Molecular Formula | C25H22O10 |
| Molecular Weight | 482.436 |
| Flash Point | 274.5±26.4 °C |
| Exact Mass | 482.121307 |
| PSA | 155.14000 |
| LogP | 2.59 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.684 |
| Storage condition | −20°C |
| Stability | Stable. Incompatible with strong oxidizing agents, strong bases. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39-S24/25-S22 |
| RIDADR | 3172 |
| WGK Germany | 3 |
| RTECS | DJ2981770 |
| HS Code | 2932999099 |
|
~67% 
Silymarin CAS#:22888-70-6 |
| Literature: Monti, Daniela; Gazak, Radek; Marhol, Petr; Biedermann, David; Purchartova, Katerina; Fedrigo, Mirko; Riva, Sergio; Kren, Vladimir Journal of Natural Products, 2010 , vol. 73, # 4 p. 613 - 619 |
|
~0% 
Silymarin CAS#:22888-70-6 
Silymarin CAS#:22888-70-6 |
| Literature: Monti, Daniela; Gazak, Radek; Marhol, Petr; Biedermann, David; Purchartova, Katerina; Fedrigo, Mirko; Riva, Sergio; Kren, Vladimir Journal of Natural Products, 2010 , vol. 73, # 4 p. 613 - 619 |
|
~% 
Silymarin CAS#:22888-70-6 |
| Literature: Archiv der Pharmazie, , vol. 316, # 5 p. 385 - 394 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
High-performance thin-layer chromatography linked with (bio)assays and mass spectrometry - a suited method for discovery and quantification of bioactive components? Exemplarily shown for turmeric and milk thistle extracts.
J. Chromatogr. A. 1394 , 137-47, (2015) Extraction parameters, chemical fingerprint, and the single compounds' activity levels were considered for the selection of active botanicals. For an initial survey, the total bioactivity (i.e., total... |
|
|
The in-vitro effect of complementary and alternative medicines on cytochrome P450 2C9 activity.
J. Pharm. Pharmacol. 66(9) , 1339-46, (2014) The aim of this study is to establish the inhibitory effects of 14 commonly used complementary and alternative medicines (CAM) on the metabolism of cytochrome P450 2C9 (CYP2C9) substrates 7-methoxy-4-... |
|
|
Effect of silibinin on the pharmacokinetics of nitrendipine in rabbits.
Eur. J. Drug Metab. Pharmacokinet. 39(4) , 277-81, (2014) Silibinin, a major constituent of silymarin, is widely used for its hepatoprotective effects. This study investigated the effect of silibinin on the pharmacokinetics of oral nitrendipine in rabbits. I... |
| flavobin |
| (2R,3R)-3,5,7-Trihydroxy-2-[(2R,3R)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydro-4H-chromen-4-one |
| EINECS 245-302-5 |
| SILYBIN A |
| 4H-1-Benzopyran-4-one, 2-[(2R,3R)-2,3-dihydro-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-1,4-benzodioxin-6-yl]-2,3-dihydro-3,5,7-trihydroxy-, (2R,3R)- |
| SILYBININ |
| [2R-[2a,3b,6(2R*,3R*)]]-2-[2,3-Dihydro-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-1,4-benzodioxin-6-yl]-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one |
| silliver |
| Silibinin |
| Silybin |
| Silybum Substance E6 |
| silybine |
| MFCD00872186 |
| 7c3mt |
| Silymrin |
| Dura-Silymarin |
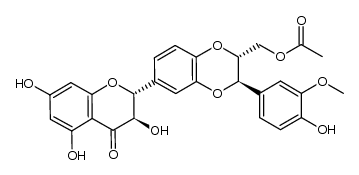
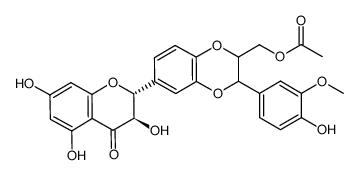
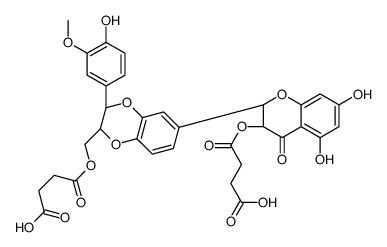
![4-(((2R,3R)-3-(4-hydroxy-3-methoxyphenyl)-6-((2R,3R)-3,5,7-trihydroxy-4-oxochroman-2-yl)-2,3-dihydrobenzo[b][1,4]dioxin-2-yl)methoxy)-4-oxobutanoic acid structure](https://image.chemsrc.com/caspic/049/86124-93-8.png)
![4-(((2R,3R)-5,7-dihydroxy-2-((2R,3R)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-oxochroman-3-yl)oxy)-4-oxobutanoic acid structure](https://image.chemsrc.com/caspic/087/86124-92-7.png)

