Taxifolin

Taxifolin structure
|
Common Name | Taxifolin | ||
|---|---|---|---|---|
| CAS Number | 480-18-2 | Molecular Weight | 304.252 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 687.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C15H12O7 | Melting Point | 230-233°C (dec.) | |
| MSDS | USA | Flash Point | 264.2±25.0 °C | |
Use of TaxifolinTaxifolin exhibits important anti-tyrosinase activity. Taxifolin exhibits significant inhibitory activity against collagenase with an IC50 value of 193.3 μM. |
| Name | (+)-taxifolin |
|---|---|
| Synonym | More Synonyms |
| Description | Taxifolin exhibits important anti-tyrosinase activity. Taxifolin exhibits significant inhibitory activity against collagenase with an IC50 value of 193.3 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 193.3 μM (Collagenase)[1] Tyrosinase[1] |
| In Vitro | This is confirmed by the investigation of pure Taxifolin and (+)-Catechin against collagenase activity. Taxifolin exhibits significant inhibitory activity with an IC50 value of 193.3 μM while (+)-Catechin is not active[1]. Taxifolin is a ubiquitous bioactive constituent of foods and herbs. Taxifolin (dihydroquercetin) is a bioactive flavanonol commonly found in grapes, citrus fruits, onions, green tea, olive oil, wine, and many other foods, as well as several herbs (such as milk thistle, French maritime bark, Douglas fir bark, and Smilacis Glabrae Rhizoma)[2]. |
| In Vivo | Taxifolin may be easily metabolized and that its metabolites are the prevalent form in vivo, although limited information is available on metabolism of Taxifolin in vivo[2]. |
| Animal Admin | Rats[2] Twelve male Sprague-Dawley rats (weighing 180-220 g) are used. The rats are randomly divided into two groups (six rats per group), a drug group and a blank group. Taxifolin is suspended in 0.5% CMC-Na solution and orally administered to the drug group at a dose of 200 mg/kg body weight, while blank group rats are orally administered 0.5% CMC-Na solution at the same volume. All rats are dosed once a day (at 9:00 a.m.) for 3 days. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 687.6±55.0 °C at 760 mmHg |
| Melting Point | 230-233°C (dec.) |
| Molecular Formula | C15H12O7 |
| Molecular Weight | 304.252 |
| Flash Point | 264.2±25.0 °C |
| Exact Mass | 304.058289 |
| PSA | 127.45000 |
| LogP | 1.82 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.763 |
| Storage condition | -20?C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | LK6920000 |
| HS Code | 2932999099 |
| Precursor 8 | |
|---|---|
| DownStream 8 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Combined experimental and in silico approaches for exploring antiperoxidative potential of structurally diverse classes of antioxidants on docetaxel-induced lipid peroxidation using 4-HNE as the model marker.
Bioorg. Chem. 56 , 1-7, (2014) The objective of the present work was tantamount to explain the antiperoxidative potential and structural requirements of twenty-eight structurally diverse classes of antioxidants on docetaxel-induced... |
|
|
In vitro effects of myricetin, morin, apigenin, (+)-taxifolin, (+)-catechin, (-)-epicatechin, naringenin and naringin on cytochrome b5 reduction by purified NADH-cytochrome b5 reductase.
Toxicology 308 , 34-40, (2013) The microsomal NADH-dependent electron transport system consisting of cytochrome b5 reductase and cytochrome b5 participates in a number of physiologically important processes including lipid metaboli... |
|
|
Chemical profiling and quantification of Gua-Lou-Gui-Zhi decoction by high performance liquid chromatography/quadrupole-time-of-flight mass spectrometry and ultra-performance liquid chromatography/triple quadrupole mass spectrometry.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 986-987 , 69-84, (2015) Gua-Lou-Gui-Zhi decoction (GLGZD) is a classical formula of traditional Chinese medicine, which has been commonly used to treat dysfunction after stroke, epilepsy and spinal cord injury. In this study... |
| EINECS 207-543-4 |
| Dihydroquercetin |
| (3R)-2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4H-chromen-4-one |
| (2R,3R)-3,3',4',5,7-Pentahydroxyflavanone |
| 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-, (2R,3R)- |
| Distylin |
| (+)-Taxifolin |
| (2S,3S)-2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4H-chromen-4-one |
| 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-, (2R-trans)- |
| 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-, (2S,3S)- |
| Taxifolin |
| MFCD00006845 |
| (-)-taxifolin |
| Taxifoliol |
| (2R,3R)-2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4H-chromen-4-one |
 CAS#:1298135-47-3
CAS#:1298135-47-3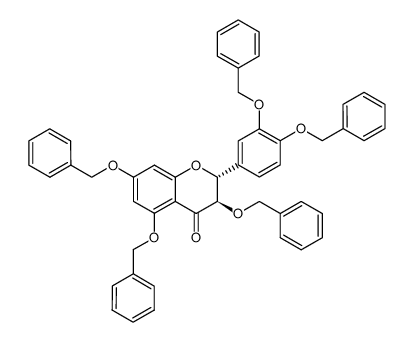 CAS#:574749-31-8
CAS#:574749-31-8 CAS#:36804-13-4
CAS#:36804-13-4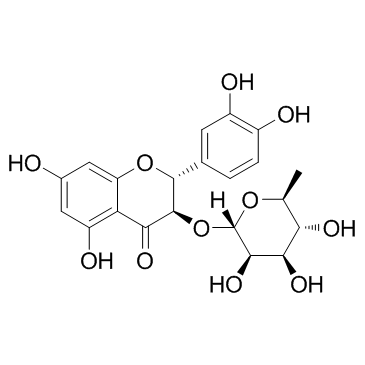 CAS#:29838-67-3
CAS#:29838-67-3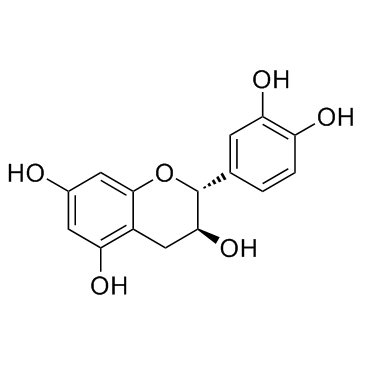 CAS#:154-23-4
CAS#:154-23-4![(2R-trans)-2-[3,4-bis(phenylmethoxy)phenyl]-3,4-dihydro-3,5,7-tris(phenylmethoxy)-2H-1-benzopyran Structure](https://image.chemsrc.com/caspic/447/85443-49-8.png) CAS#:85443-49-8
CAS#:85443-49-8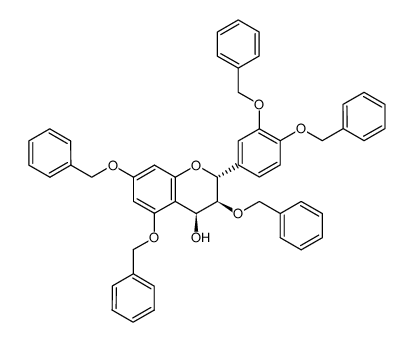 CAS#:574749-29-4
CAS#:574749-29-4 CAS#:40672-47-7
CAS#:40672-47-7 CAS#:855-97-0
CAS#:855-97-0 CAS#:117-39-5
CAS#:117-39-5 CAS#:552-58-9
CAS#:552-58-9 CAS#:23567-23-9
CAS#:23567-23-9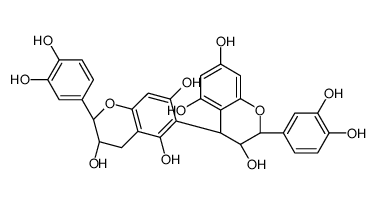 CAS#:12798-57-1
CAS#:12798-57-1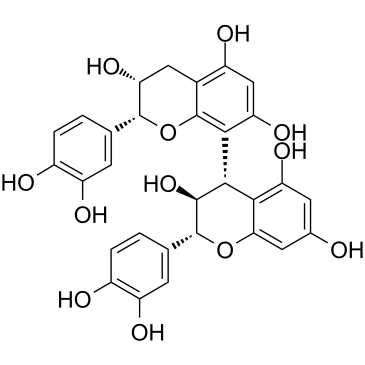 CAS#:29106-51-2
CAS#:29106-51-2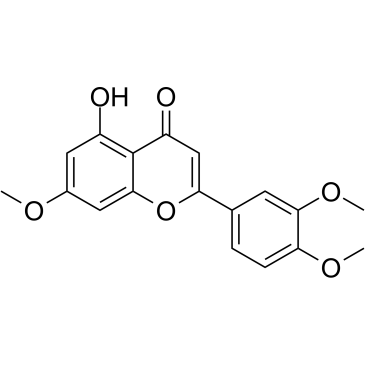 CAS#:29080-58-8
CAS#:29080-58-8
