Baicalein
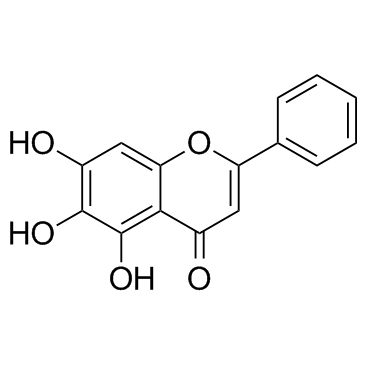
Baicalein structure
|
Common Name | Baicalein | ||
|---|---|---|---|---|
| CAS Number | 491-67-8 | Molecular Weight | 270.237 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 575.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C15H10O5 | Melting Point | 256-271 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 225.3±23.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of BaicaleinBaicalein (5,6,7-Trihydroxyflavone) is a xanthine oxidase inhibitor with an IC50 value of 3.12 mM. |
| Name | baicalein |
|---|---|
| Synonym | More Synonyms |
| Description | Baicalein (5,6,7-Trihydroxyflavone) is a xanthine oxidase inhibitor with an IC50 value of 3.12 mM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 3.12 mM (xanthine oxidase)[1] |
| In Vitro | Baicalein suppresses mitogen induced T cell proliferation and cytokine secretion in vitro. Pre-treatment with baicalein significantly suppresses Con A or anti-CD3/CD28 mAb induced proliferation as well as cytokine secretion at 25 μM. Baicalein treatment induces DNA binding of NF-κB but inhibits thioredoxin activity in the nuclear compartment[2]. Baicalein suppresses proliferation, migration, and invasion of MDA-MB-231 cells in a time- and dose-dependent manner. Baicalein significantly decreases the expression of SATB1 in MDA-MB-231 cells. Baicalein also downregulates the expression of Wnt1 and β-catenin proteins and transcription level of Wnt/β-catenin-targeted genes[3]. |
| In Vivo | Baicalein suppresses induction of graft versus host disease but does not inhibit homeostatic proliferation of T-cells in mice. This observation clearly shows potent anti-inflammatory activity of baicalein in vivo[2]. Rats treated with baicalein are protected against an increase in heart to body weight ratio, plasma level of brain natriuretic peptides, intraventricular septum thickness, myocardial collagen volume of left ventricle (all P<0.05, respectively). The antifibrotic effects of baicalein are further illustrated by the suppressed expression of left ventricle pro-collagens I and III accompanied by the decreased expression of 12-lipoxygenase, and by reduced expression and activity of matrix metallopeptidase 9 and extracellular signal-regulated kinases. Baicalein can inhibit cardiac fibrosis in hypertensive rats[4]. |
| Cell Assay | MTT assay is conducted to evaluate the effect of baicalein on proliferation of breast cancer cells. MDA-MB-231 cells are routinely digested, collected, and then seeded in 96-well plates at a density of 8×103 cells/well. After incubation for 12-24 hours, cells are treated with 0, 20, 40, 60, 80, 100, and 120 μM baicalein according to their experimental grouping and then incubated at 37°C for 24, 48, and 72 hours[3]. |
| Animal Admin | Rats: Baicalein is suspended in 1% methylcellulose. Rats are treated with baicalein suspension via oral garvage. SHR and WKY rats are divided into 4 groups (n=8 per group): 12-week treatment with high-dose (200 mg/kg/day) or low-dose (50 mg/kg/day) group; and 4-week treatment with high-dose or low-dose group. The 12-week and 4-week negative control groups of SHR and WKY rats (n=8 per group) receive vehicle while positive control groups (Val group, n=8 per group) receive valsartan (20 mg/kg/day) for comparison[4]. Mice: To study the in vivo anti-inflammatory efficacy of baicalein, graft-versus-host disease (GVHD) model is used. Splenic lymphocytes from C57BL/6 mice are incubated with baicalein in vitro (25 μM, 4h) and adoptively transferred to immune-compromised Balb/c mice[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 575.9±50.0 °C at 760 mmHg |
| Melting Point | 256-271 °C(lit.) |
| Molecular Formula | C15H10O5 |
| Molecular Weight | 270.237 |
| Flash Point | 225.3±23.6 °C |
| Exact Mass | 270.052826 |
| PSA | 90.90000 |
| LogP | 3.31 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.732 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2932999099 |
| Precursor 10 | |
|---|---|
| DownStream 8 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
A nicotinic receptor-mediated anti-inflammatory effect of the flavonoid rhamnetin in BV2 microglia.
Fitoterapia 98 , 11-21, (2014) The alpha7 nicotinic acetylcholine receptor (nAChR) is a potential target in neuroinflammation. Screening a plant extract library identified Solidago nemoralis as containing methyl-quercetin derivativ... |
|
|
Identification and quantification of phytochemicals in nutraceutical products from green tea by UHPLC-Orbitrap-MS.
Food Chem. 173 , 607-18, (2014) A method has been developed and validated for the simultaneous detection and quantification of phytochemicals in nutraceutical products obtained from green tea. For that purpose, ultra-high performanc... |
|
|
In vitro sensitization of erythrocytes to programmed cell death following baicalein treatment.
Toxins (Basel.) 6(9) , 2771-86, (2014) The polyphenolic flavonoid Baicalein has been shown to trigger suicidal death or apoptosis of tumor cells and is thus considered for the prevention and treatment of malignancy. Similar to apoptosis of... |
| 4H-1-BENZOPYRAN-4-ONE,5,6,7-TRIHYDROXY-2-PHENYL |
| Noroxylin |
| 5,6,7-TRIHYDROXYFLAVONE |
| Baicalein |
| MFCD00017459 |
| 5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one |
| Baicelein |
| Baicalin |
 CAS#:973-67-1
CAS#:973-67-1 CAS#:29550-13-8
CAS#:29550-13-8 CAS#:21967-41-9
CAS#:21967-41-9 CAS#:480-11-5
CAS#:480-11-5 CAS#:671791-94-9
CAS#:671791-94-9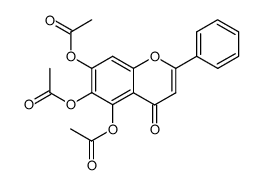 CAS#:67047-05-6
CAS#:67047-05-6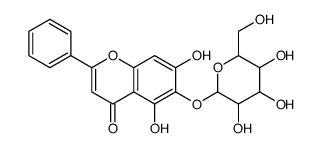 CAS#:28279-72-3
CAS#:28279-72-3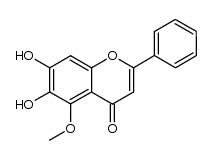 CAS#:150036-33-2
CAS#:150036-33-2 CAS#:642-71-7
CAS#:642-71-7 CAS#:412027-80-6
CAS#:412027-80-6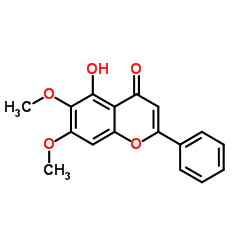 CAS#:740-33-0
CAS#:740-33-0 CAS#:2035-05-4
CAS#:2035-05-4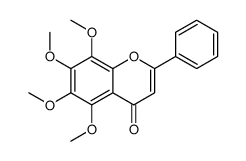 CAS#:3162-43-4
CAS#:3162-43-4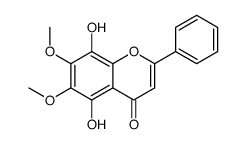 CAS#:73202-52-5
CAS#:73202-52-5
