6-(Furfurylamino)purine
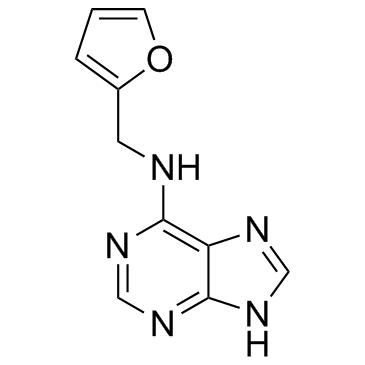
6-(Furfurylamino)purine structure
|
Common Name | 6-(Furfurylamino)purine | ||
|---|---|---|---|---|
| CAS Number | 525-79-1 | Molecular Weight | 215.211 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 367.6±52.0 °C at 760 mmHg | |
| Molecular Formula | C10H9N5O | Melting Point | 269-271 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 176.1±30.7 °C | |
Use of 6-(Furfurylamino)purineKinetin (N6-furfuryladenine) belongs to a group of plant growth hormones involved in cell division, differentiation and other physiological processes.IC50 Value: Target:Kinetin is one of the widely used components in numerous skin care cosmetics and cosmeceuticals, such as Valeant products kinerase. Recently, kinetin has the potential to be a treatment for the human splicing disease familial dysautonomia.in vitro: Kinetin-induced cell death reflected by the morphological changes of nuclei including their invagination, volume increase, chromatin condensation and degradation as well as formation of micronuclei showed by AO/EB and 4,6-diamidino-2-phenylindol staining was accompanied by changes including increase in conductivity of cell electrolytes secreted to culture media, decrease in the number of the G1- and G2-phase cells and appearance of fraction of hypoploid cells as the effect of DNA degradation without ladder formation [1]. The plant cytokinin kinetin dramatically increases exon 20 inclusion in RNA isolated from cultured FD cells [3].in vivo: Subjects received 23.5 mg/Kg/d for 28 d. An increase in WT IKBKAP mRNA expression in leukocytes was noted after 8 d in six of eight individuals; after 28 d, the mean increase compared with baseline was significant (p = 0.002) [2].Toxicity: On mice with leukaemia P388, kinetin has no effect on the tumour growth, and it appears to be toxic at the dose of 25 mg/kg [4]. |
| Name | kinetin |
|---|---|
| Synonym | More Synonyms |
| Description | Kinetin (N6-furfuryladenine) belongs to a group of plant growth hormones involved in cell division, differentiation and other physiological processes.IC50 Value: Target:Kinetin is one of the widely used components in numerous skin care cosmetics and cosmeceuticals, such as Valeant products kinerase. Recently, kinetin has the potential to be a treatment for the human splicing disease familial dysautonomia.in vitro: Kinetin-induced cell death reflected by the morphological changes of nuclei including their invagination, volume increase, chromatin condensation and degradation as well as formation of micronuclei showed by AO/EB and 4,6-diamidino-2-phenylindol staining was accompanied by changes including increase in conductivity of cell electrolytes secreted to culture media, decrease in the number of the G1- and G2-phase cells and appearance of fraction of hypoploid cells as the effect of DNA degradation without ladder formation [1]. The plant cytokinin kinetin dramatically increases exon 20 inclusion in RNA isolated from cultured FD cells [3].in vivo: Subjects received 23.5 mg/Kg/d for 28 d. An increase in WT IKBKAP mRNA expression in leukocytes was noted after 8 d in six of eight individuals; after 28 d, the mean increase compared with baseline was significant (p = 0.002) [2].Toxicity: On mice with leukaemia P388, kinetin has no effect on the tumour growth, and it appears to be toxic at the dose of 25 mg/kg [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 367.6±52.0 °C at 760 mmHg |
| Melting Point | 269-271 °C (dec.)(lit.) |
| Molecular Formula | C10H9N5O |
| Molecular Weight | 215.211 |
| Flash Point | 176.1±30.7 °C |
| Exact Mass | 215.080704 |
| PSA | 79.63000 |
| LogP | -0.56 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.774 |
| Storage condition | −20°C |
| Water Solubility | H2O: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | 68-36/37/38 |
| Safety Phrases | S23-S24/25-S22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | AU6270000 |
| HS Code | 29349990 |
| Precursor 9 | |
|---|---|
| DownStream 8 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Cloning and Expression of TNF Related Apoptosis Inducing Ligand in Nicotiana tabacum.
Iran. J. Pharm. Res. 14(1) , 189-201, (2015) Molecular farming has been considered as a secure and economical approach for production of biopharmaceuticals. Human TNF Related Apoptosis Inducing Ligand (TRAIL) as a promising biopharmaceutical can... |
|
|
Evaluation of Antioxidant and Antibacterial Potentials of Nigella sativa L. Suspension Cultures under Elicitation.
Biomed Res. Int. 2015 , 708691, (2015) Nigella sativa L. (family Ranunculaceae) is an annual herb of immense medicinal properties because of its major active components (i.e., thymoquinone (TQ), thymohydroquinone (THQ), and thymol (THY)). ... |
|
|
[Study on optimization of induction system of test-tube tuberous roots from leaves of Rehmannia glutinosa].
Zhongguo Zhong Yao Za Zhi 37(24) , 3812-4, (2012) To study the effect of sucrose and plant growth substances of different concentrations on the induction of test-tube tuberous roots of Rehmannia glutinosa, in order to establish an efficient system fo... |
| 6-Furfurylaminopurine,N6-Furfuryladenine |
| N-(2-Furylmethyl)-7H-purin-6-amine |
| N-furfuryl-7H-purin-6-amine |
| N-(2-Furylmethyl)-3H-purin-6-amine |
| N-(Furan-2-ylmethyl)-1H-purin-6-amine |
| MFCD00075757 |
| 7H-Purin-6-amine, N-(2-furanylmethyl)- |
| T56 BM DN FN HNJ IM1- BT5OJ |
| cytokinin |
| fap |
| EINECS 208-382-2 |
| N-(2-furanylmethyl)-1H-purin-6-amine |
| QUINETINE |
| 6-(Furfurylamino)purine |
| Kinetin |
| N-Furfuryl-Adenine |
| Kiniten |
| N6-Furfuryladenine |
| N-(Furan-2-ylmethyl)-9H-purin-6-amine |
| 3H-Purin-6-amine, N-(2-furanylmethyl)- |
| 6-Furfurylaminopurine |
| KINETINE |
| N6-(2-Furylmethyl)-9H-purin-6-amine |
| N(Sup6)-Furfuryladenine |
| N-(furan-2-ylmethyl)-7H-purin-6-amine |
| Adenine, N-furfuryl- |
| N-Furfuryladenine |
| Kinetina |
| T56 BM DN GN INJ FM1- BT5OJ |
 CAS#:98-01-1
CAS#:98-01-1 CAS#:66224-66-6
CAS#:66224-66-6 CAS#:617-89-0
CAS#:617-89-0 CAS#:56025-87-7
CAS#:56025-87-7 CAS#:68-94-0
CAS#:68-94-0 CAS#:19769-32-5
CAS#:19769-32-5 CAS#:50-66-8
CAS#:50-66-8 CAS#:98-00-0
CAS#:98-00-0![[5-(6-acetamidopurin-9-yl)-3,4-diacetyloxy-oxolan-2-yl]methyl acetate Structure](https://image.chemsrc.com/caspic/032/7387-58-8.png) CAS#:7387-58-8
CAS#:7387-58-8 CAS#:109403-64-7
CAS#:109403-64-7 CAS#:4338-47-0
CAS#:4338-47-0 CAS#:124-20-9
CAS#:124-20-9 CAS#:71-44-3
CAS#:71-44-3 CAS#:110-60-1
CAS#:110-60-1
