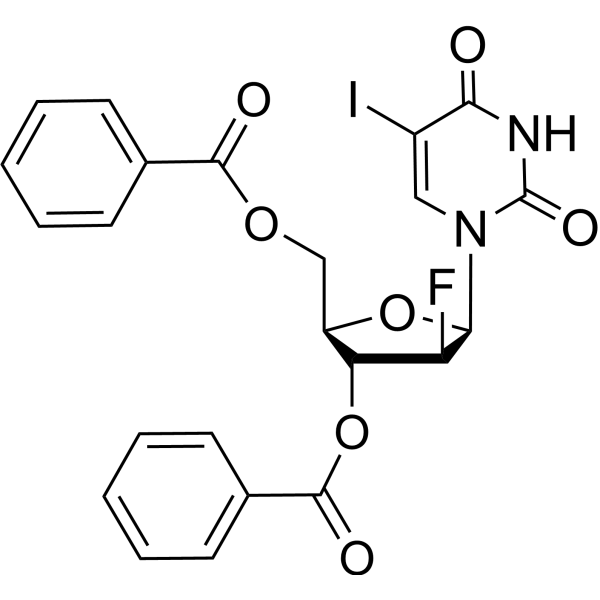
Fialuridine
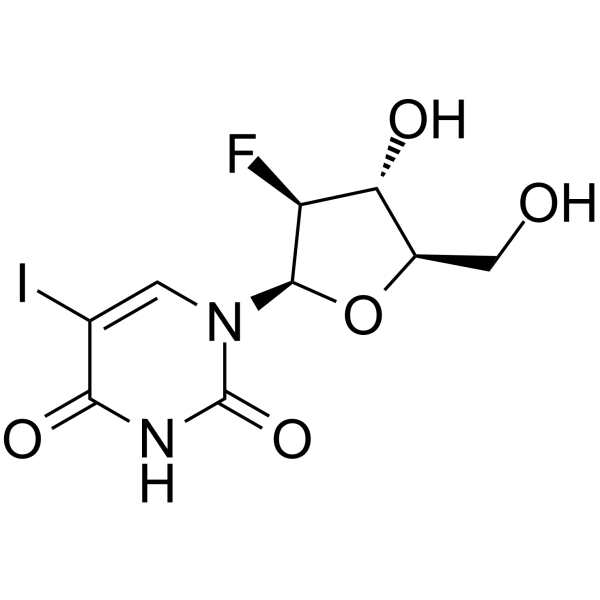
Fialuridine structure
|
Common Name | Fialuridine | ||
|---|---|---|---|---|
| CAS Number | 69123-98-4 | Molecular Weight | 372.09 | |
| Density | 2.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C9H10FIN2O5 | Melting Point | 216-217ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of FialuridineFialuridine is a nucleoside analog with antiviral activity[1]. |
| Name | 1-(2-Deoxy-2-fluoro-β-D-arabinofuranosyl)-5-iodouracil |
|---|---|
| Synonym | More Synonyms |
| Description | Fialuridine is a nucleoside analog with antiviral activity[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 2.2±0.1 g/cm3 |
|---|---|
| Melting Point | 216-217ºC |
| Molecular Formula | C9H10FIN2O5 |
| Molecular Weight | 372.09 |
| Exact Mass | 371.961823 |
| PSA | 104.55000 |
| LogP | -0.32 |
| Index of Refraction | 1.685 |
| Storage condition | Refrigerator |
| Water Solubility | DMSO: soluble5mg/mL, clear (warmed) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
~94% 
Fialuridine CAS#:69123-98-4 |
| Literature: RESprotech GmbH Patent: US2010/227834 A1, 2010 ; Location in patent: Page/Page column 18 ; |
|
~% 
Fialuridine CAS#:69123-98-4 |
| Literature: US4211773 A1, ; |
|
~% 
Fialuridine CAS#:69123-98-4 |
| Literature: Journal of Organic Chemistry, , vol. 73, # 21 p. 8236 - 8243 |
|
~% 
Fialuridine CAS#:69123-98-4 |
| Literature: Journal of Labelled Compounds and Radiopharmaceuticals, , vol. 40, p. 91 - 93 |
|
~% 
Fialuridine CAS#:69123-98-4 |
| Literature: Journal of Organic Chemistry, , vol. 50, # 19 p. 3644 - 3647 |
|
~% 
Fialuridine CAS#:69123-98-4 |
| Literature: Journal of Organic Chemistry, , vol. 50, # 19 p. 3644 - 3647 |
|
~% 
Fialuridine CAS#:69123-98-4 |
| Literature: Journal of Labelled Compounds and Radiopharmaceuticals, , vol. 40, p. 91 - 93 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Improved synthesis of 2'-deoxy-2'-[18F]-fluoro-1-beta-D-arabinofuranosyl-5-iodouracil ([18F]-FIAU).
Nucl. Med. Biol. 37(4) , 439-42, (2010) An improved synthesis of 2'-[(18)F]-fluoro-2'-deoxy-1-beta-D-arabinofuranosyl-5-iodouracil ([(18)F]-FIAU) has been developed. The method utilizes trimethylsilyl trifluoromethanesulfonate (TMSOTf) cata... |
|
|
Imaging of a localized bacterial infection with endogenous thymidine kinase using radioisotope-labeled nucleosides.
Int. J. Med. Microbiol. 302(2) , 101-7, (2012) The importance of noninvasive imaging methods to bacterial infections is widely recognized. To obtain bacterial infection imaging with radioisotope-labeled nucleosides, bacterial thymidine kinase (tk)... |
|
|
Enhanced antitumor effects by combination gene therapy using MDR1 gene shRNA and HSV1-tk in a xenograft mouse model.
Cancer Lett. 291(1) , 83-9, (2010) The use of a novel therapeutic vector containing HSV1-thymidine kinase (HSV1-tk) and a short hairpin RNA for the MDR1 gene (shMDR) was proposed previously. We investigated the antitumor effects in an ... |
| 1-(2-Deoxy-2-fluoro-β-D-arabinofuranosyl)-5-iodouracil |
| 1-[(2R,3S,4R,5R)-3-Fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidine-2,4-dione |
| 1-(2-Deoxy-2-fluoro-b-D-arabinofuranosyl)-5-iodo-2,4(1H,3H)-pyrimidinedione |
| 1-(2-deoxy-2-fluoro-b-D-arabinofuranosyl)-5-iodopyrimidine-2,4(1H,3H)-dione |
| Fialuridine |
| 5-Iodo-2'-fluoroarauracil |
| 1-(2-Deoxy-2-fluoro-β-D-arabinofuranosyl)-5-iodopyrimidine-2,4(1H,3H)-dione |
| 2,4(1H,3H)-pyrimidinedione, 1-(2-deoxy-2-fluoro-b-D-arabinofuranosyl)-5-iodo- |
| 2'-Deoxy-2'-fluoro-5-iodouridine |
| 1-(2-Deoxy-2-fluoro-β-D-arabinofuranosyl)-5-iodo-2,4(1H,3H)-pyrimidinedione |
| 1-(2-Deoxy-2-fluoro-b-D-arabinofuranosyl)-5-iodouracil |
| 2,4(1H,3H)-Pyrimidinedione, 1-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)-5-iodo- |