Xanthoxylin
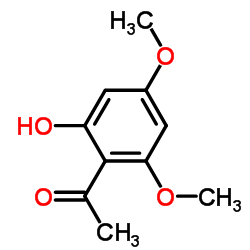
Xanthoxylin structure
|
Common Name | Xanthoxylin | ||
|---|---|---|---|---|
| CAS Number | 90-24-4 | Molecular Weight | 196.200 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 355.1±37.0 °C at 760 mmHg | |
| Molecular Formula | C10H12O4 | Melting Point | 80-84 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 141.2±20.0 °C | |
Use of XanthoxylinXanthoxylin (Xanthoxyline) is isolated from Zanthoxylum simulans. Xanthoxylin (Xanthoxyline) has antifungal and antispasmodic activities[1][2]. |
| Name | 4',6'-Dimethoxy-2'-hydroxyacetophenone |
|---|---|
| Synonym | More Synonyms |
| Description | Xanthoxylin (Xanthoxyline) is isolated from Zanthoxylum simulans. Xanthoxylin (Xanthoxyline) has antifungal and antispasmodic activities[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 355.1±37.0 °C at 760 mmHg |
| Melting Point | 80-84 °C(lit.) |
| Molecular Formula | C10H12O4 |
| Molecular Weight | 196.200 |
| Flash Point | 141.2±20.0 °C |
| Exact Mass | 196.073563 |
| PSA | 55.76000 |
| LogP | 2.37 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.528 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914509090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Collateral sensitivity of resistant MRP1-overexpressing cells to flavonoids and derivatives through GSH efflux.
Biochem. Pharmacol. 90(3) , 235-45, (2014) The multidrug resistance protein 1 (MRP1) is involved in multidrug resistance of cancer cells by mediating drug efflux out of cells, often in co-transport with glutathione (GSH). GSH efflux mediated b... |
|
|
Antinociceptive action of 2-(4-bromobenzoyl)-3-methyl-4,6-dimethoxy benzofuran, a novel xanthoxyline derivative on chemical and thermal models of nociception in mice.
J. Pharmacol. Exp. Ther. 278(1) , 304-12, (1996) The antinociceptive effect of the novel xanthoxyline derivative 2-(4-bromobenzoyl)-3-methyl-4-6-dimethoxy benzofuran) (BMDB), given i.p., p.o., s.c., subplantarly, intrathecally or by i.c.v. routes wa... |
|
|
Synthesis of chalcone analogues with increased antileishmanial activity.
Bioorg. Med. Chem. 14(5) , 1538-45, (2006) Eighteen analogues of an active natural chalcone were synthesized using xanthoxyline and some derivatives, and these analogues were tested for selective activity against both promastigotes and intrace... |
| 2-HYDROXY-4,6-DIMETHOXYACETOPHENONE |
| MFCD00017243 |
| Brevifolin (VAN) |
| EINECS 201-978-3 |
| 4,6-Dimethoxy-2-hydroxyacetophenone |
| Phloracetophenone 4,6-Dimethyl Ether |
| 2,4-Dimethoxy-6-hydroxyacetophenone |
| Acetophenone, 2'-hydroxy-4',6'-dimethoxy- |
| Ethanone, 1-(2-hydroxy-4,6-dimethoxyphenyl)- |
| 1-(2-Hydroxy-4,6-dimethoxyphenyl)ethanone |
 CAS#:480-66-0
CAS#:480-66-0 CAS#:77-78-1
CAS#:77-78-1 CAS#:500-99-2
CAS#:500-99-2 CAS#:75-36-5
CAS#:75-36-5 CAS#:832-58-6
CAS#:832-58-6 CAS#:621-23-8
CAS#:621-23-8 CAS#:2142-68-9
CAS#:2142-68-9 CAS#:7087-68-5
CAS#:7087-68-5 CAS#:80-48-8
CAS#:80-48-8 CAS#:74-88-4
CAS#:74-88-4 CAS#:10496-67-0
CAS#:10496-67-0 CAS#:10176-71-3
CAS#:10176-71-3 CAS#:3420-72-2
CAS#:3420-72-2 CAS#:3602-54-8
CAS#:3602-54-8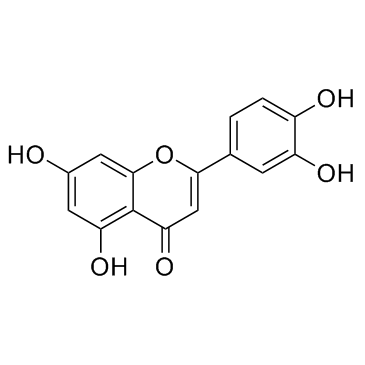 CAS#:491-70-3
CAS#:491-70-3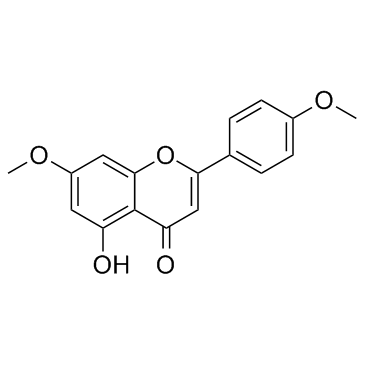 CAS#:5128-44-9
CAS#:5128-44-9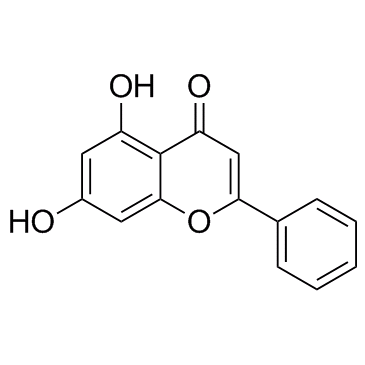 CAS#:480-40-0
CAS#:480-40-0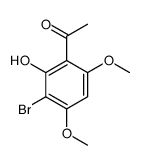 CAS#:18064-89-6
CAS#:18064-89-6
