932-53-6
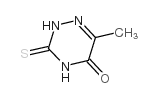
| Name | 6-methyl-2H-1,2,4-triazine-3,5-dione |
|---|---|
| Synonyms |
6-Methyl-2H-[1,2,4]triazin-3,5-dion
USAF CB-28 1,2,4-triazine-3,5-diol, 6-methyl- Azathymine 6-methyl-2H-[1,2,4]triazine-3,5-dione Azathymine,6 6-azathymine 1,2,4-Triazine-3,5(2H,4H)-dione,6-methyl 6-methyl-2H-[1,2,4]triazin-3,5-dione 6-methyl-2,3,4,5-tetrahydro-1,2,4-triazine-3,5-dione 6-Methyl-1,2,4-triazine-3,5(2H,4H)-dione 6-methyl-1,2,4-triazine-3,5-diol 5-Methyl-6-azauracil MFCD00006457 1,2,4-Triazine-3,5(2H,4H)-dione, 6-methyl- EINECS 213-253-9 |
| Description | 6-Azathymine, a 6-nitrogen analog of thymine, is a potent D-3-aminoisobutyrate-pyruvate aminotransferase inhibitor. 6-Azathymine inhibits the biosynthesis of DNA, and has antibacterial and antiviral activities[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | 6-Azathymine is a competitive antagonist of the growth of Streptococcus faecalis (8043) and several other strains of microorganisms. Studies of the mechanism of action of 6-Azathymine reveal that S. faecalis can convert the analog to the corresponding deoxyriboside, azathymidine[2]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Melting Point | 210-212°C |
| Molecular Formula | C4H5N3O2 |
| Molecular Weight | 127.101 |
| Exact Mass | 127.038177 |
| PSA | 78.61000 |
| LogP | -2.05 |
| Index of Refraction | 1.692 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Safety Phrases | S22-S24/25 |
|---|---|
| RTECS | XY8050000 |
| HS Code | 2933699090 |
|
~79% 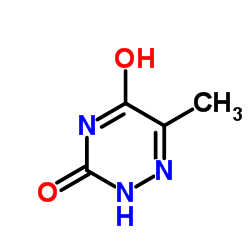
932-53-6 |
| Literature: Koegler, Martin; Busson, Roger; De Jonghe, Steven; Rozenski, Jef; Van Belle, Kristien; Louat, Thierry; Munier-Lehmann, Helne; Herdewijn, Piet Chemistry and Biodiversity, 2012 , vol. 9, # 3 p. 536 - 556 |
|
~% 
932-53-6 |
| Literature: Monatshefte fur Chemie, , vol. 139, # 12 p. 1483 - 1490 |
| Precursor 2 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933699090 |
|---|---|
| Summary | 2933699090 other compounds containing an unfused triazine ring (whether or not hydrogenated) in the structure。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:20.0% |