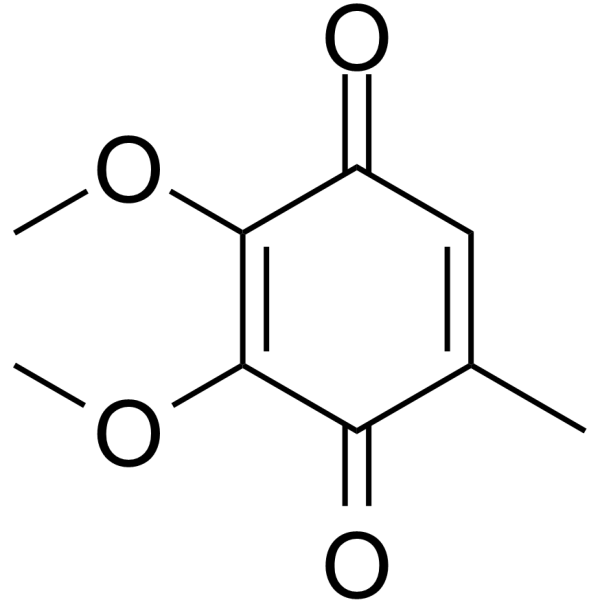
605-94-7
| Name | ubiquinone-0 |
|---|---|
| Synonyms |
1,2,3-Dimethoxy-5-methyl-1,4-benzoquinone
2,5-Cyclohexadiene-1,4-dione, 2,3-dimethoxy-5-methyl- EINECS 210-100-8 2,3-dimethoxy-5-methyl-1,4-benzoquinone 2,3-Dimethoxy-5-methylcyclohexa-2,5-dien-1,4-dion p-Benzoquinone, 2,3-dimethoxy-5-methyl- 2,3-Dimethoxy-5-Methyl-p-Benzoquinone Ubiquinone Q0 2,3-dimethoxy-5-methylcyclohexa-2,5-diene-1,4-dione MFCD00001595 |
| Description | Coenzyme Q0 (CoQ0) is a potent, oral active ubiquinone compound can be derived from Antrodia cinnamomea. Coenzyme Q0 induces apoptosis and autophagy, suppresses of HER-2/AKT/mTOR signaling to potentiate the apoptosis and autophagy mechanisms. Coenzyme Q0 regulates NFκB/AP-1 activation and enhances Nrf2 stabilization in attenuation of inflammation and redox imbalance. Coenzyme Q0 has anti-angiogenic activity through downregulation of MMP-9/NF-κB and upregulation of HO-1 signaling[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Coenzyme Q0 (0-40 µM; 24 h) and inhibits viability and growth of human ovarian carcinoma cells[1]. Coenzyme Q0 (CoQ0) (0-30 µM; 24 h; SKOV-3 cells) has anti-proliferative activity through induction of G2/M cell-cycle arrest and reduction of cell-cycle regulatory proteins[1]. Coenzyme Q0 (CoQ0) (0-30 µM; 0-30 min; SKOV-3 cells) increases intracellular ROS levels to promote SKOV-3 cell death[1]. Coenzyme Q0 (CoQ0) (0-30 µM; 24 h; SKOV-3 cells) induces autophagy by increase accumulation of LC3-II, GFP-LC3 puncta, AVOs formation and Beclin-1/Bcl-2 dysregulation[1]. Coenzyme Q0 (CoQ0) (0-30 µM; 24 h; SKOV-3 cells) induces apoptosis by mitochondrial (caspase-3, PARP and Bax/Bcl-2 dysregulation) and ER stress (caspase-12 and Hsp70) signals[1]. Coenzyme Q0 (CoQ0) (30 µM; 24 h; SKOV-3 cells) suppresses of HER-2/AKT/mTOR signaling to potentiate the apoptosis and autophagy mechanisms[1]. Coenzyme Q0 (CoQ0) (0-10 µM; 0.5-18 h; RAW264.7 cells) regulates NFκB/AP-1 activation and enhances Nrf2 stabilization[2]. Coenzyme Q0 (CoQ0) (5 µM; 0-12 h; EA.hy 926 cells) has anti-angiogenic activity in EA.hy 926 cells[3]. Cell Viability Assay[1] Cell Line: SKOV-3, A2780 and A2870/CP70 cells Concentration: 0, 10, 20, 30 and 40 µM Incubation Time: 24 hours Result: Decreased viability with the IC50 values of 26.6 µM, 27.3 µM and 28.4 µM for SKOV-3, A2780 and A2870/CP70 cells, respectively. Cell Cycle Analysis[1] Cell Line: SKOV-3, A2780 and A2870/CP70 cells Concentration: 0, 10, 20 and 30 µM Incubation Time: 24 hours Result: Arrested cell cycle at G2/M phase and reduced cell-cycle proteins in SKOV-3 cells. Apoptosis Analysis[1] Cell Line: SKOV-3, A2780 and A2870/CP70 cells Concentration: 0, 5, 15 and 30 µM Incubation Time: 24 hours Result: Promoted the conversion of LC3–1 to LC3-II and increased the LC3-II accumulation. Increased Bax/Bcl-2 ratio in a dose-dependent manner. Apoptosis Analysis[1] Cell Line: SKOV-3 cells Concentration: 0, 10, 20 and 30 µM Incubation Time: 24 hours Result: Had the percentage of early apoptotic cells are 25.1%, 34% and 36% for 10, 20 and 30 µM, respectively. Western Blot Analysis[1] Cell Line: SKOV-3 cells Concentration: 0, 5, 15 and 30 µM Incubation Time: 24 hours Result: Activated of caspase-3 and cleavaged of PARP. Increased the expressions of caspase-12, HSP-70 and Bax in a dose-dependent manner, decreased the expressions of Bcl-2. Western Blot Analysis[1] Cell Line: SKOV-3 cells Concentration: 30 µM Incubation Time: 24 hours Result: Decreased the phosphorylated HER-2 (Y1221) levels, p-AKT (Ser473) and p-mTOR (S2448) levels. Western Blot Analysis[2] Cell Line: RAW264.7 cells Concentration: 0, 2.5, 5 and 10 µM Incubation Time: 0.5-18 hours Result: Inhibited iNOS/COX-2 protein expressions with reductions of NO, PGE2, TNF-α and IL-1β secretions. Western Blot Analysis[3] Cell Line: EA.hy 926 cells Concentration: 5 µM Incubation Time: 0, 1, 3, 6 and 12 hours Result: Increased expressions of heme oxygenase-1 (HO-1) and γ-glutamylcysteine synthetase (γ-GCLC), inhibits protein expressions of matrix metalloproteinase-9 (MMP-9), reduces TNF-α-induced nuclear translocation and transcriptional activation of nuclear factor-κB (NF-κB). |
| In Vivo | Coenzyme Q0 (CoQ0) (1.5 and 2.5 mg/kg; i.p.; once every four days, for 52 d) suppresses tumor growth in SKOV-3 xenografted nude mice[1]. Coenzyme Q0(CoQ0) (5 mg/kg; p.o.; for 4 h) has anti-inflammatory activities through Nrf2 activation and NFκB inhibition in liver and spleen of LPS-treated mice[2]. Animal Model: SKOV-3 xenografted nude mice[1] Dosage: 1.5 and 2.5 mg/kg Administration: Intraperitoneal injection; Once every four days, for 52 days Result: Inhibited the tumor growth at 1.5 and 2.5 mg/kg. Animal Model: LPS-treated female FVB mice[2] Dosage: 5 mg/kg Administration: Oral administration; for 4 hours Result: Down-regulates inflammatory genes in liver and spleen tissues of LPS injected mice. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 331.4±42.0 °C at 760 mmHg |
| Melting Point | 58-60 °C(lit.) |
| Molecular Formula | C9H10O4 |
| Molecular Weight | 182.173 |
| Flash Point | 148.6±27.9 °C |
| Exact Mass | 182.057907 |
| PSA | 52.60000 |
| LogP | 0.12 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.498 |
| Storage condition | 2-8°C |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, reducing agents. |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |