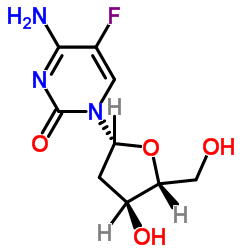
50-91-9
| Name | 5-fluoro-2'-deoxyuridine |
|---|---|
| Synonyms |
1-(2-Deoxy-β-D-erythro-pentofuranosyl)-5-fluorpyrimidin-2,4(1H,3H)-dion
5-Fluoro-2'-deoxyuridine 1-(2-Deoxy-β-D-ribofuranosyl)-5-fluorouracil 5-fluoro-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxyméthyl)tétrahydrofuran-2-yl]pyrimidine-2,4(1H,3H)-dione (+)-5-Fluoro-2-deoxyuridine Floxuridin (+)-5-Fluoro-2‘-deoxyuridine 2(1H)-Pyrimidinone, 1-(2-deoxy-β-D-erythro-pentofuranosyl)-5-fluoro-4-hydroxy- Floxuridinum Floxuridine 5-Fluoro-2'-deoxy-b-uridine 5-Fluoro-2'-deoxy-β-uridine (+)-5-fluoro-2′-deoxyuridine Fluorodeoxyuridine Deoxyfluorouridine 2'-Deoxy-5-fluorouridine 5-fluoro-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione Fluoruridine deoxyribose 5-Fluorodeoxyuridine 5-Fluoro-1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione EINECS 200-072-5 Uridine, 2'-deoxy-5-fluoro- 5-Fluoro-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydro-2-furanyl]-2,4(1H,3H)-pyrimidinedione (+)-5-Fluoro-2'-deoxyuridine MFCD00006530 1-(2-Deoxy-β-D-erythro-pentofuranosyl)-5-fluoro-4-hydroxy-2(1H)-pyrimidinone 5-Fluor-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2,4(1H,3H)-dion 1-(2-Deoxy-b-D-ribofuranosyl)-5-fluorouracil 5-fluoro-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidine-2,4(1H,3H)-dione 2′-Deoxy-5-fluorouridine FUDR |
| Description | Floxuridine (5-fluorodeoxyuridine) is an oncology drug that belongs to the class known as antimetabolites with an GI50 of 5.1 μM for the inhibition of PEPT1. IC50 value: Target: Nucleoside antimetabolite/analogFloxuridine (Fludara) is a prodrug of floxuridine and an oncology agent with an GI50 of 5.1 μM for the inhibition of MDCK/PEPT1. Floxuridine (Fludara) belongs to the class known as antimetabolites. Floxuridine (Fludara) is most often used in the treatment of colorectal cancer. Floxuridine, an analog of 5-fluorouracil, is a fluorinated pyrimidine. Floxuridine (Fludara) works because it is broken down by the body into its active form, which is the same as a metabolite of 5-Fluorouracil [1]. FdUrd induced an immediate increase in tumor uptake of 5-[(125)I]iodo-2'-deoxyuridine, that vanished after 6 h, as also confirmed by flow cytometry. Biodistribution measurements showed that FdUrd pretreatment increased [(18)F]FLT uptake in all tumors by factors of 3.2 to 7.8 compared with controls, while [(18)F]FDG tumor uptake was about fourfold and sixfold lower in breast cancers and lymphoma. Dynamic PET in FdUrd pretreated mice showed that [(18)F]FLT uptake in all tumors increased steadily up to 1.5 h. MRI showed a well-vascularized homogenous lymphoma with high [(18)F]FLT uptake, while in breast cancer, a central necrosis shown by MRI was inactive in PET, consistent with the histomorphological analysis [2].Clinical indications: Colorectal tumor; Liver tumorFDA Approved Date: December 1970 |
|---|---|
| Related Catalog | |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 483.0±55.0 °C at 760 mmHg |
| Melting Point | 148 °C(lit.) |
| Molecular Formula | C9H11FN2O5 |
| Molecular Weight | 246.192 |
| Flash Point | 245.9±31.5 °C |
| Exact Mass | 246.065201 |
| PSA | 104.55000 |
| LogP | -1.22 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.676 |
| Storage condition | 2~8°C |
| Water Solubility | soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36/37/38;R68 |
| Safety Phrases | 22-36-36/37/39-26 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | YU7525000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2934999090 |
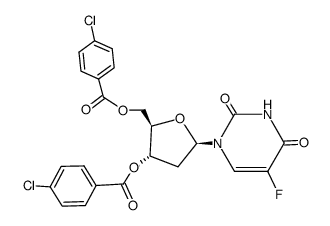
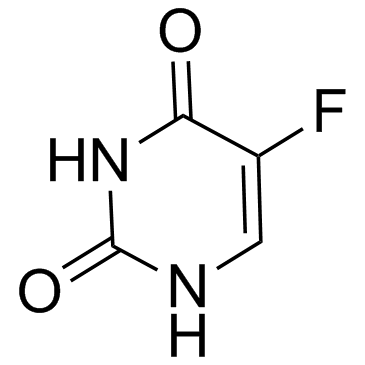
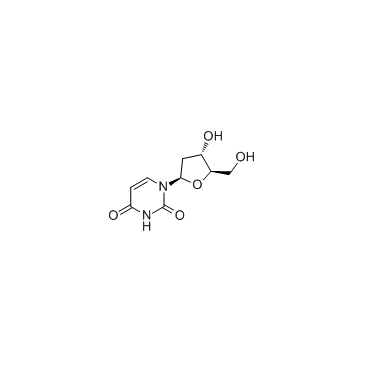
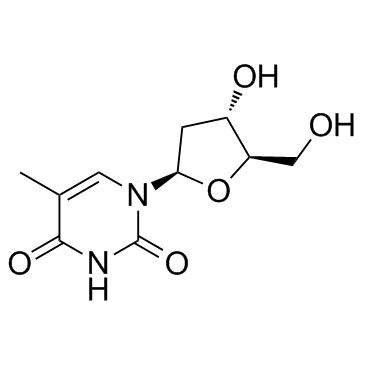
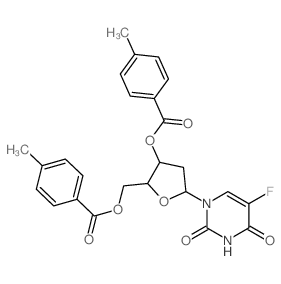
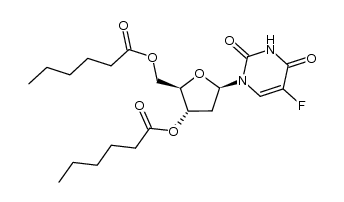
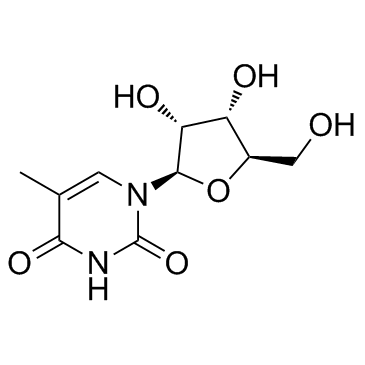
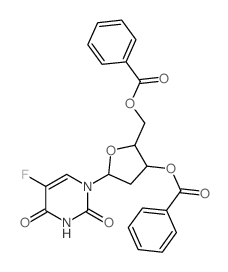
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |


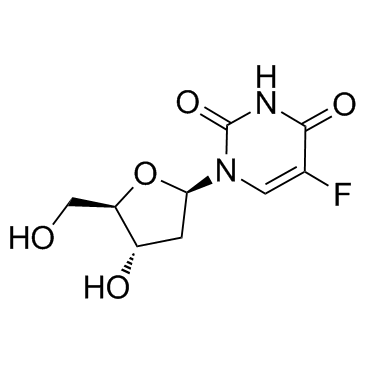
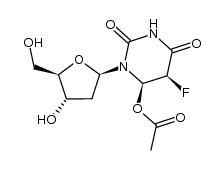
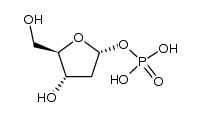
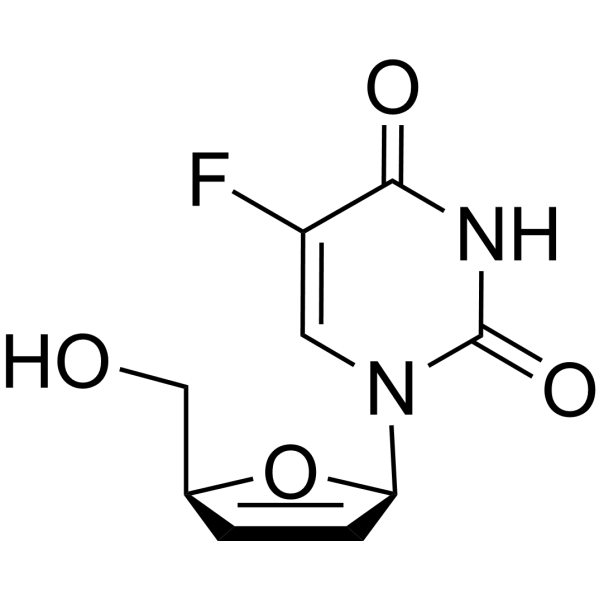

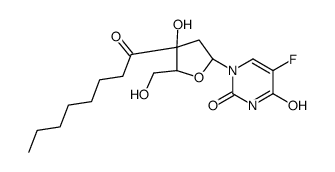
![[(2R,3R,4R,5R)-5-(5-fluoro-2,4-dioxo-pyrimidin-1-yl)-3,4-dihydroxy-oxo lan-2-yl]methyl octanoate structure](https://image.chemsrc.com/caspic/325/118694-10-3.png)